GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (3)
This article will continue to introduce the GPCR (G Protein-Coupled Receptor) targets and pipelines that garnered attention for Roche and TEVA at the JP Morgan Healthcare Conference 2024.For more details, please follow the link below.
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (1)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (2)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (4)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (5)
Roche in JPM 2024
Roche's continuous innovation in pharmaceuticals and diagnostics as well as ongoing improvements to industry standards. The company has not only transformed the standard of care for a variety of diseases by launching numerous blockbuster drugs, but it has also established leadership positions in several new areas.

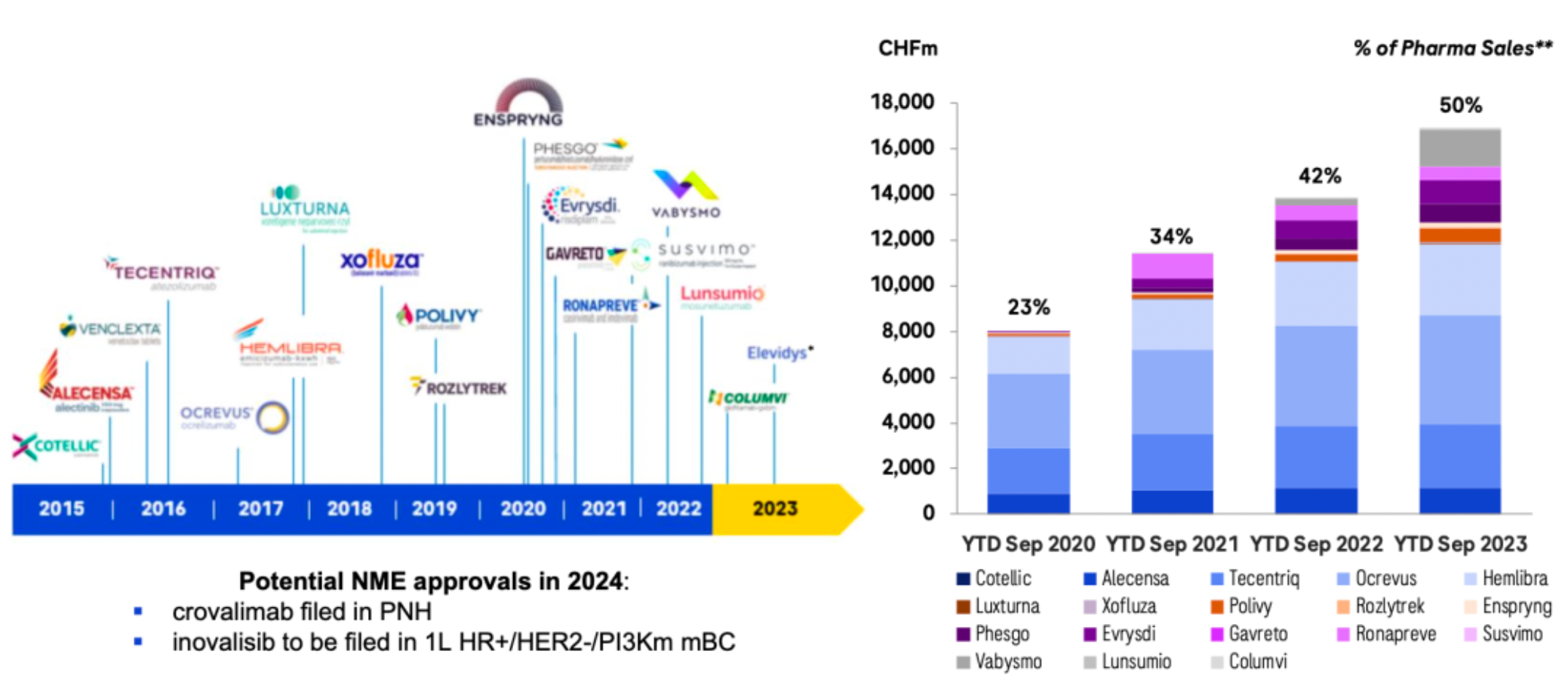
The company's newly marketed and diversified product portfolio continues to drive growth in the pharmaceutical sector, maintaining a historic momentum of new drug launches, with two new drugs approved in 2023.
Advance multiple key R&D areas to accelerate the delivery speed of pharmaceuticals. Set ambitious R&D goals; enhance the product portfolio framework; reform product portfolio management and governance; obtain the best external innovations; optimize the R&D engine and invest in emerging technologies; develop talent, culture, and mindsets to achieve objectives.
Roche is fully prepared for the new pharmaceutical industry model in the artificial intelligence era.
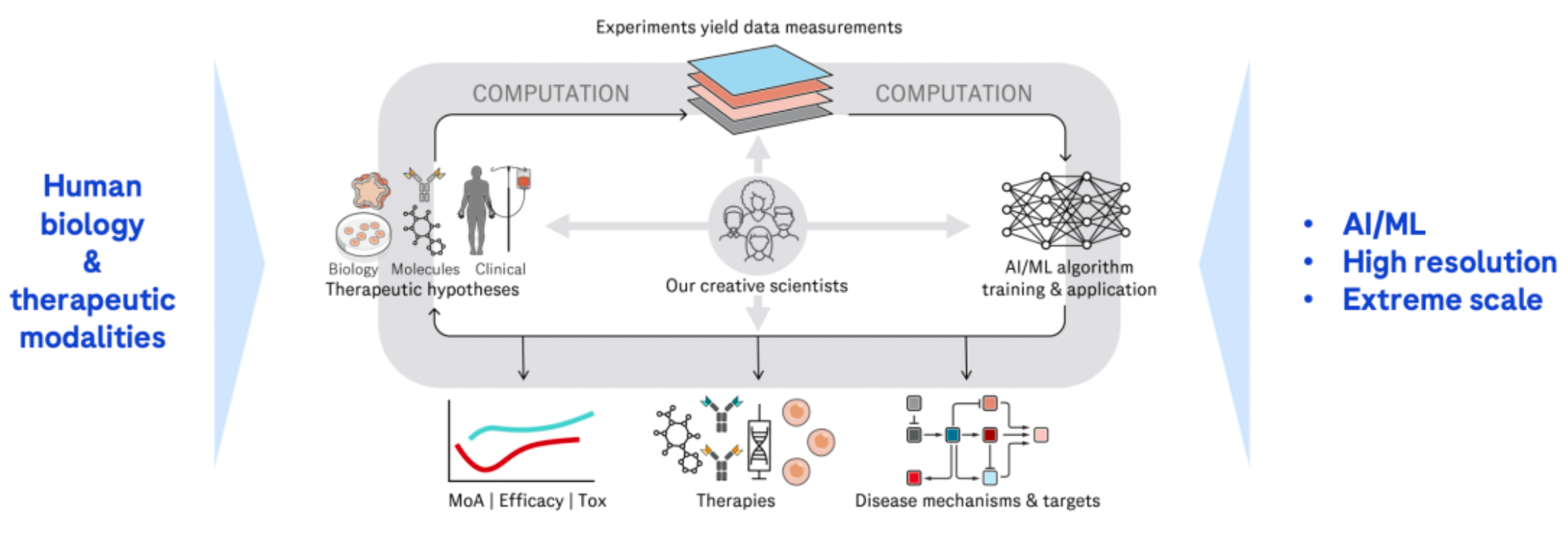
Through sustained external collaborations, it hastens the advancement of an innovative product pipeline. Over the past two years, an increasing number of transactions have focused on non-prescription drugs at the clinical stage. The main investments during this period have centered on oncology/hematology and cardiovascular/metabolic disorders.
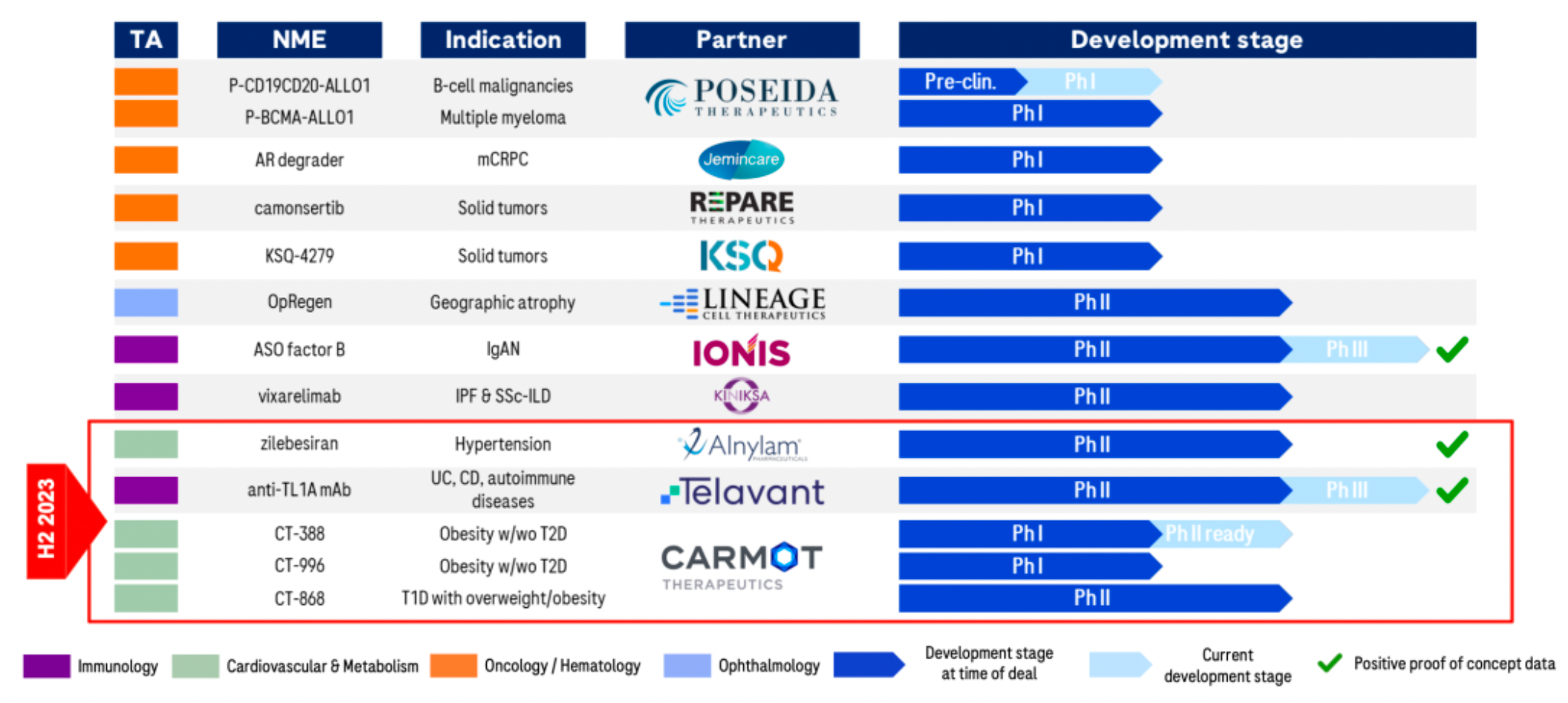
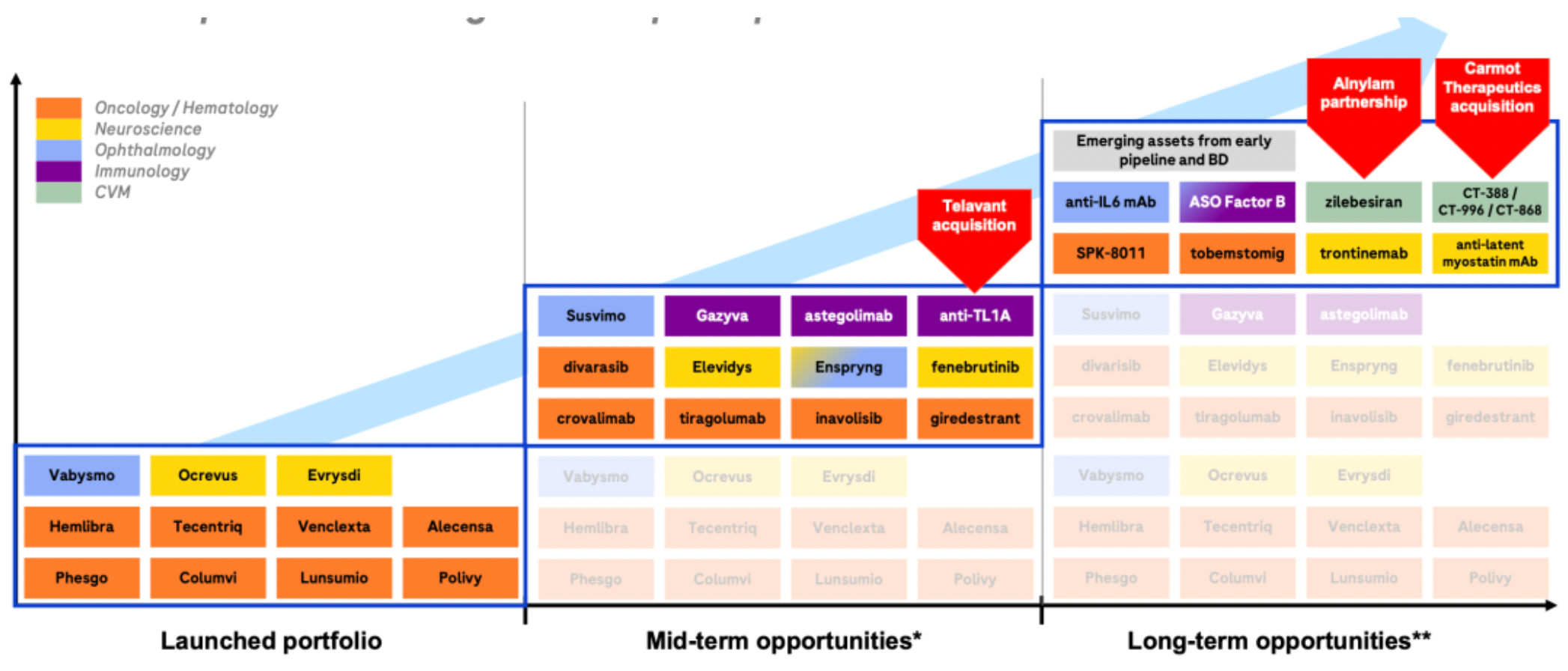
A recent series of business operations have laid the foundation for rapid development over the next 6-7 years. The company has formed several major clinical candidate products.
Crovalimab is a monoclonal antibody targeting the complement component C5 protein. C5 is a part of the complement system that, upon activation, participates in thrombus formation and inflammatory responses. The mechanism of action of Crovalimab involves inhibiting the cleavage of C5 into C5a and C5b, thereby preventing complement-mediated hemolysis. Indications for Crovalimab include paroxysmal nocturnal hemoglobinuria (PNH) and other complement-mediated autoimmune diseases. C5aR is a member of the complement peptide receptors belonging to the Class A GPCR family, which mainly comprises three members: C3aR, C5aR1, and C5aR2.
CT-388 is a dual agonist synthetic peptide targeting the GIPR and GLP-1R receptors. In early December 2023, Roche announced a definitive merger agreement to acquire the American company Carmot Therapeutics, including three clinical-stage assets with leading potential in the obesity and diabetes sectors: CT-388 (a once-weekly subcutaneous injection, GLP-1/GIP dual receptor agonist), CT-996 (a daily oral, small-molecule GLP-1 receptor agonist), and CT-868 (a daily subcutaneous injection, GLP-1/GIP dual receptor agonist). For this, Roche will pay a $2.7 billion upfront payment, as well as up to $400 million in milestone payments.
TEVA in JPM 2024
TEVA is an Israeli multinational pharmaceutical company that launched the "Pivot to Growth Strategy" in May 2023, marking a shift from a historical trend of declining revenues to a restoration of revenue growth, with a focus on growth assets and pipeline development.
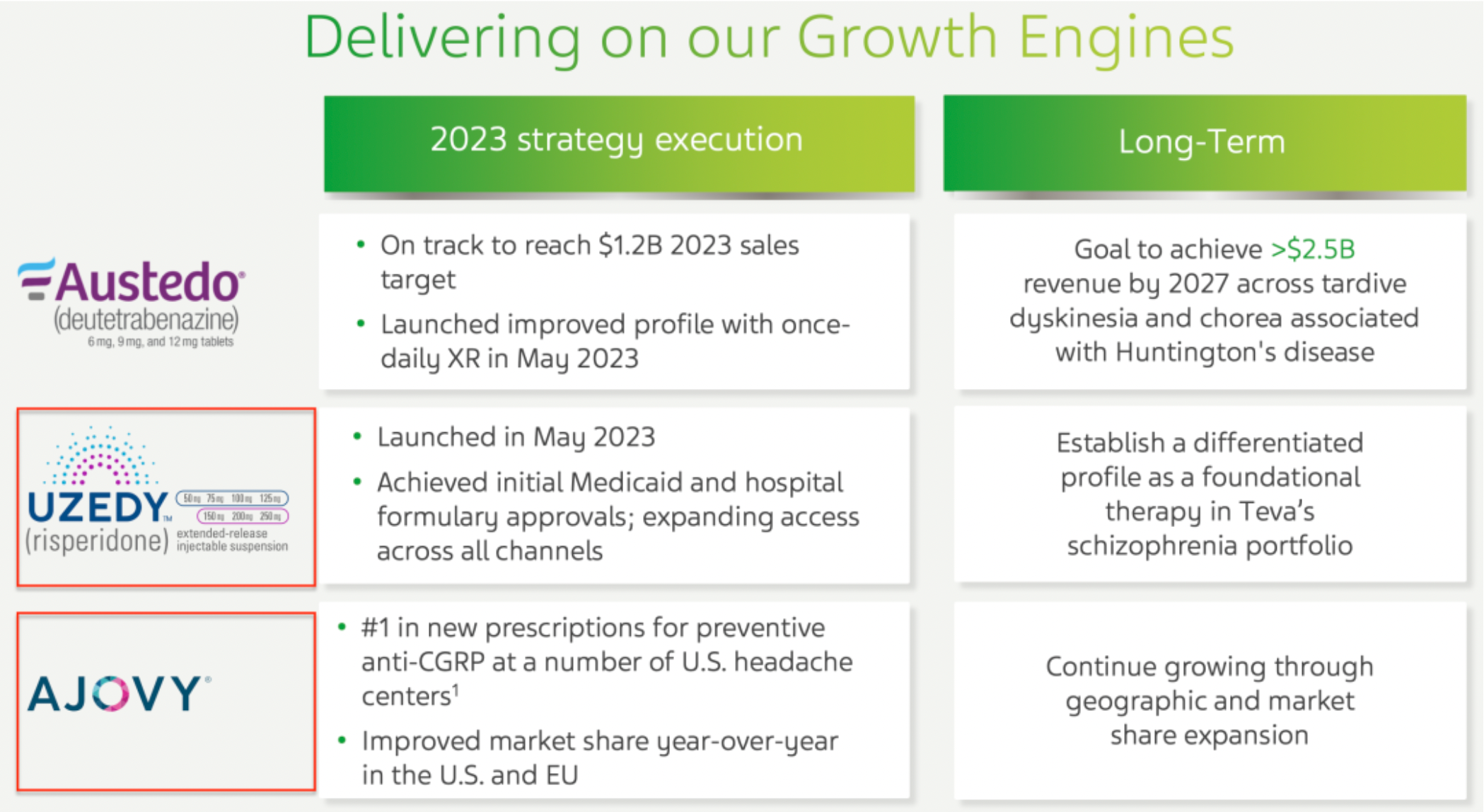
The company's range of products, such as AUSTEDO® and AJOVY®, have matured and become important drivers of the company's growth; the company has made significant progress in the development of late-stage clinical products, including collaboration with external partners on anti-TL1A therapy. The generics business has returned to a state of revenue growth; at the same time, through various initiatives to accelerate the development of new drugs and improve overall innovation capabilities.
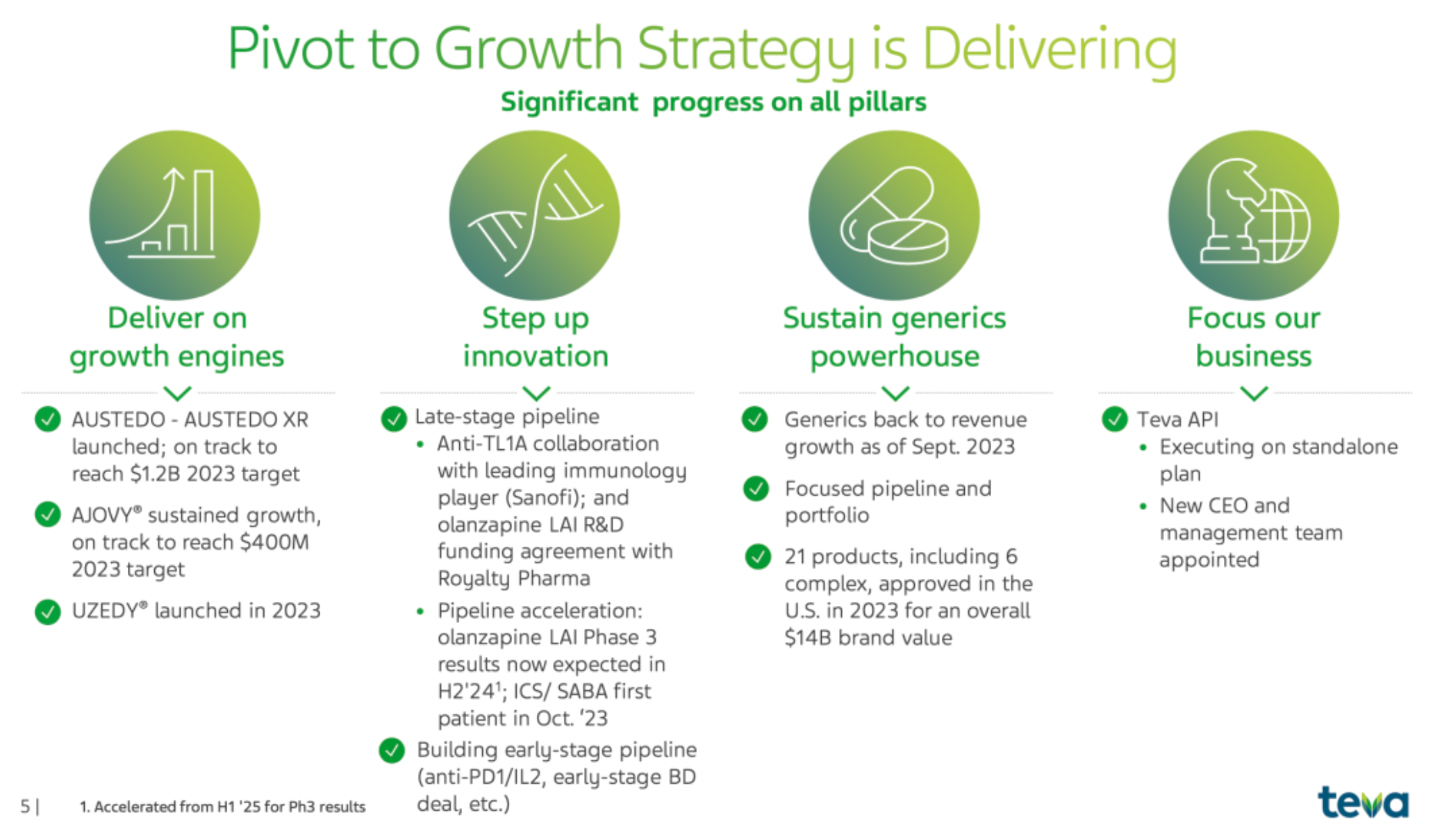
The company has increased its market share in the United States and European Union compared to last year, especially within the schizophrenia product portfolio, where it is dedicated to establishing a differentiated product position to stand out as cornerstone therapy. The results of the phase III clinical trial of the company's long-acting olanzapine LAI are expected to be released in the second half of 2024, ahead of schedule; the ICS/SABA project began enrolling its first patient in October 2023. Additionally, the new drug the company launched in May 2023 has ranked first in new prescriptions within the preventive anti-CGRP therapy field at multiple headache centers in the United States.
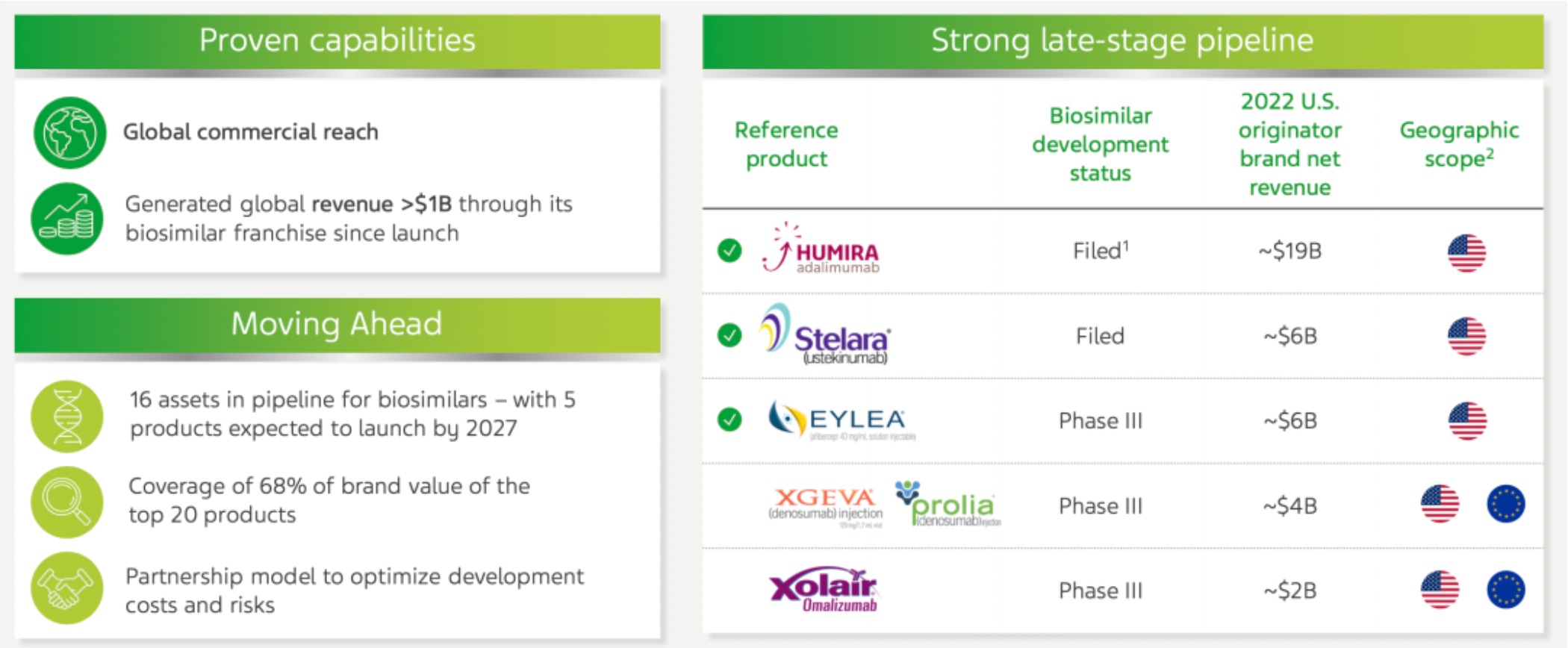
In the field of biosimilar business, the company has generated over 1 billion US dollars in global sales revenue through its biosimilar product line since its launch and is actively expanding its presence in this area. Currently, there are 16 pipeline assets under development, with an expectation of launching 5 products by the year 2027. These products are targeted at approximately 68% of the brand value among the top 20 global blockbuster drugs. The company employs a collaborative model to optimize research and development costs and reduce risks. This is particularly true in the development of biosimilars, where the company is advancing projects in collaboration with partners such as Alvotech.
Risperidone was approved for market release in May 2023 as an antagonist of the 5-HT2A receptors and D2 dopamine receptors, making it a second-generation antipsychotic medication. It is indicated for the treatment of a variety of psychiatric disorders, including schizophrenia, bipolar mania, psychosis, and as an adjunctive treatment for major depressive disorder.
AJOVY (Fremanezumab-VFRM) is ranked as the number one preventative anti-CGRP new prescription in multiple headache centers in the United States. Fremanezumab is a monoclonal antibody targeting CGRP, the endogenous agonist for CGRP receptors. Clinical study data has shown that directly inhibiting CGRP or antagonizing the activity of CGRP receptors is beneficial for the clinical management of migraines.
Olanzapine long-acting injection (olanzapine LAI, TEV-749) is a once-monthly subcutaneous long-acting formulation of the atypical antipsychotic drug olanzapine, currently in Phase 3 clinical trials for the treatment of schizophrenia. It has the potential to become the first long-acting olanzapine with a good safety profile. Olanzapine targets multiple receptors, acting as an antagonist of 5-HT2A, 5-HT2C, 5-HT3 receptors, and D2 dopamine receptors. Over the past 20 years, more than 100 clinical studies have been conducted on olanzapine, expanding its clinical indications to include nausea, vomiting, depression, treatment-resistant depression, bipolar disorder, and related conditions.
Teriparatide (Forteo®) is a peptide-based biosimilar drug, acting as a PTH1 receptor agonist, indicated for the clinical treatment of osteoporosis.
Octreotide (Sandostatin® LAR) is also a peptide-based biosimilar drug, functioning as an agonist of SSTR (somatostatin receptors), used to treat acromegaly as well as diarrhea associated with metastatic carcinoid tumors and vasoactive intestinal peptide-secreting tumors.
Naloxone nasal spray (Narcan®) is an opioid receptor (μ-OR) antagonist, intended for the rapid reversal of opioid overdose. Naloxone is also used as an abuse-deterrent additive in certain pharmaceutical formulations to prevent injection.