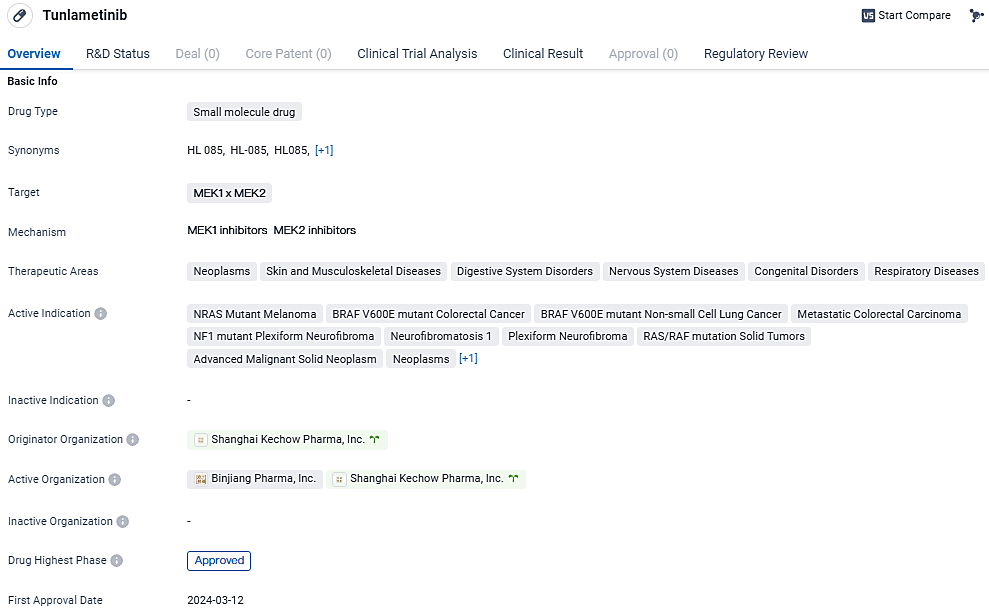
Tunlametinib (HL-085): First Targeted Therapy for Advanced Melanoma with NRAS Mutation Approved Post PD-1/PD-L1 Treatment
KeChow Pharma, a pharmaceutical enterprise at the commercialization phase, specializing in the creation and market launch of unique small molecule drugs aimed at oncological conditions, has disclosed that tunlametinib (HL-085) received official authorization from the China National Medical Products Administration. This approval is specifically for the use of tunlametinib as a therapy for individuals with advanced melanoma associated with NRAS mutations, who have undergone prior treatment with PD-1/PD-L1 inhibitors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
KeChow has achieved a significant breakthrough with the authorization of its first treatment tailored to a specific group of patients, developed by its own R&D since the company was founded. “KeChow has hit a groundbreaking achievement with the Chinese authorization of Tunlametinib – our debut internally crafted and developed compound,” Dr. Hongqi Tian, the Founder, CEO, and Chairman of KeChow stated with enthusiasm and pride.
Expounding further, Dr. Tian remarked, “Granting tunlametinib’s approval marks a crucial step in offering a new therapeutic avenue for Chinese patients affected by the NRAS genetic alteration in advanced melanoma cases who have already undergone PD-1/PD-L1 treatment. Our heartfelt thanks go to the medical professionals and patients who were an integral part of our clinical studies. Our gratitude also extends to the healthcare officials for their unwavering support. Our dedication is unwavering to ensure the prompt availability of tunlametinib to the patients in need within China.”
“Tunlametinib’s global commercialization rights are held exclusively by KeChow. Our forward-looking clinical development strategy includes thorough assessments of tunlametinib both as an independent treatment and in synergy with other established treatments. The focus of these trials spans a variety of cancers such as melanoma, neurofibromatosis type 1-associated plexiform neurofibromas, colorectal cancer, and non-small cell lung cancer, all within the Chinese market,” Dr. Tian disclosed.
Melanoma ranks amongst the most prevalent skin malignancies, with China witnessing roughly 20,000 new cases annually. NRAS mutation occurs in approximately 10.4 to 12.6% of Chinese melanoma patients, a number slightly lower compared to the global incidence of 15-25%. Patients with the NRAS mutation face a more tenacious form of melanoma with less favorable prognoses. Until now, no targeted treatments have been specifically sanctioned for NRAS-mutated melanoma. This makes Tunlametinib a pioneering small molecule pharmaceutical for this particular condition.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
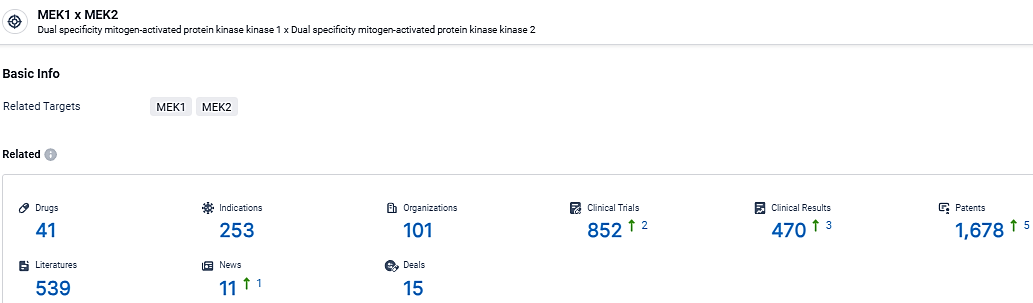
According to the data provided by the Synapse Database, As of March 19, 2024, there are 41 investigational drugs for the MEK target, including 253 indications, 101 R&D institutions involved, with related clinical trials reaching 852, and as many as 1678 patents.
Tunlametinib is a small molecule inhibitor of Mitogen-activated protein kinase kinase (MEK) discovered and developed by KeChow. Compared to other approved MEK inhibitors, tunlametinib demonstrated stronger target inhibition and improved pharmacokinetic properties. Its first approval was obtained in China in March 2024, and it has undergone priority review and conditional marketing approval.