Formycon has declared the submission of FYB203, a biosimilar candidate for Eylea® (Aflibercept), to the EMA
Formycon AG, in collaboration with its licensing associate Klinge Biopharma GmbH, has declared the submission of the commercial application for FYB203 to the European Medicines Agency. FYB203 is a biosimilar contender for Eylea®1 (its key component being Aflibercept).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Eylea® has been utilized as a therapeutic method for neovascular age-related macular degeneration and other severe eye-related diseases. It interferes with the operation of the vascular endothelial growth factor (VEGF), which is directly linked to the excessive production of blood vessels in the retina. From January to September 2023, Eylea's global sales reached a figure of roughly US$ 7 billion, demonstrating its position as the most preferable drug in anti-VEGF therapies at present.
Formycon CEO Dr. Stefan Glombitza expressed, "The recent MAA submission highlights the remarkable performance and dependability of our entire #TeamFormycon. We're happy to declare that with FYB203 as our second biosimilar candidate in the field of ophthalmology, it's predicted to gain more importance in the upcoming years due to the increasing older population."
Formycon is recognized as a leading independent firm focusing on the creation of high-grade biopharmaceutical drugs, particularly biosimilars. It concentrates on remedial solutions in ophthalmology, immunology, and other primary chronic illnesses, handling the complete value chain from technological advancement to clinical stage III and the preparation of marketing approval files.
Formycon is making a significant impact by providing as many patients as possible with access to necessary and cost-effective medicines via its biosimilars. Currently, six biosimilars are under development by Formycon. Drawing upon its vast experience in biopharmaceutical drug creation, the company is also engaged in the development of a COVID-19 treatment, FYB207.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
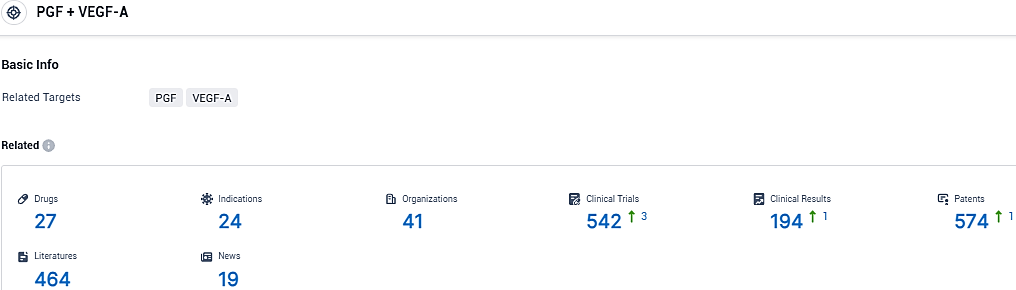
According to the data provided by the Synapse Database, As of November 30, 2023, there are 27 investigational drugs for the PGF and VEGF-A target, including 24 indications, 41 R&D institutions involved, with related clinical trials reaching 542, and as many as 574 patents.
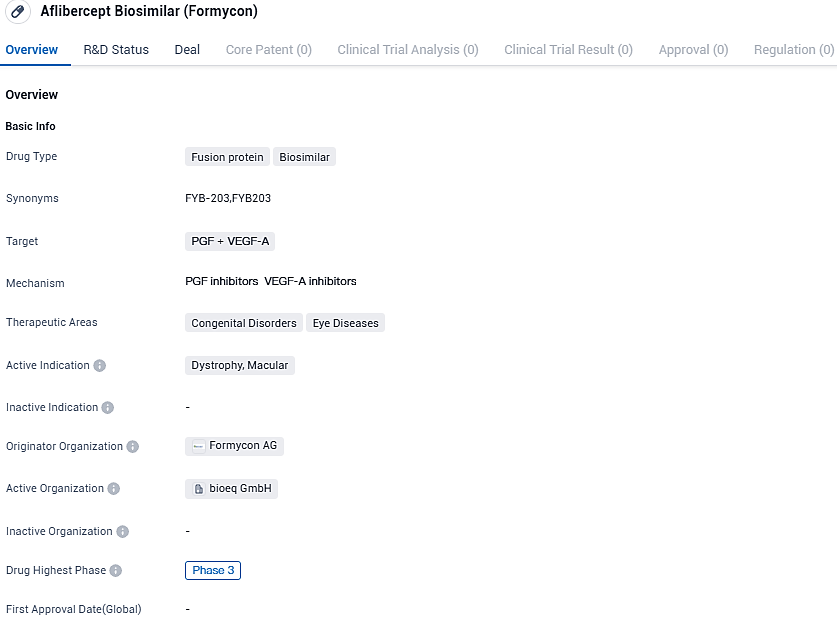
Aflibercept Biosimilar (Formycon) is a fusion protein biosimilar drug that targets PGF and VEGF-A. With its therapeutic focus on congenital disorders and eye diseases, particularly macular dystrophy, Aflibercept Biosimilar shows promise in providing potential benefits to patients in need. However, further research and clinical trials are necessary to establish its safety and efficacy before it can be made available to the public.