What are KRAS G12C inhibitors and how do you quickly get the latest development progress?
KRAS G12C is a specific mutation of the KRAS gene that plays a crucial role in the development and progression of certain cancers. This mutation occurs in the KRAS protein, which is involved in regulating cell growth and division. In the human body, the KRAS G12C mutation leads to the overactivation of signaling pathways that promote uncontrolled cell proliferation and survival. This mutation is particularly prevalent in lung and colorectal cancers, making it an attractive target for therapeutic interventions. Efforts are underway to develop drugs that specifically inhibit the KRAS G12C mutation, with the aim of effectively treating these types of cancers.
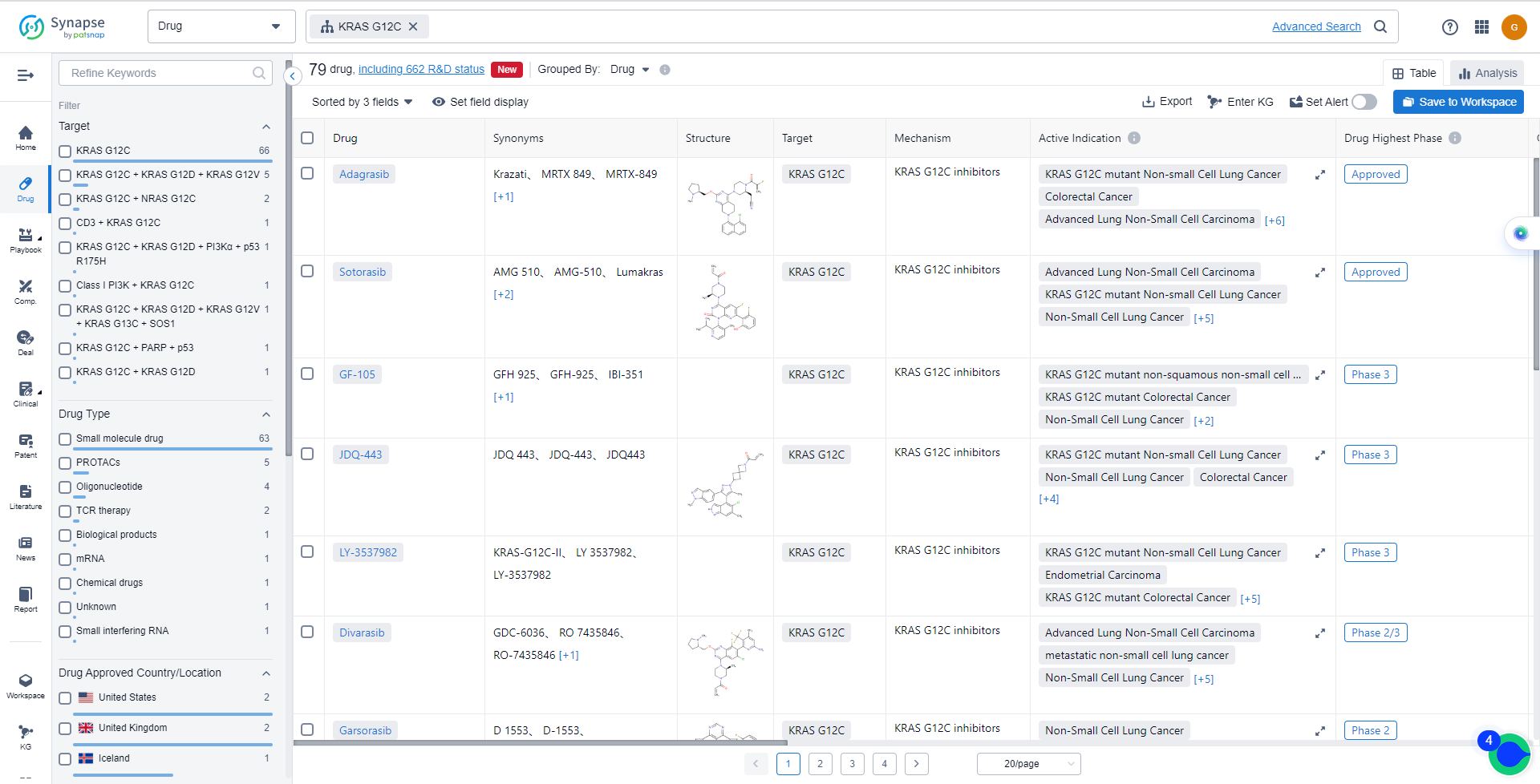
KRAS G12C inhibitors are a class of drugs that target the KRAS G12C mutation, a common oncogene in human cancers. The development of these inhibitors began with the discovery of a cysteine drug-binding pocket in the GDP-bound mutant KRAS G12C protein. After decades of efforts, three covalent G12C-specific inhibitors from three independent companies have recently entered clinical trials.
The first KRAS G12C inhibitor to reach clinical trials was sotorasib. The decision to advance the molecule that would become sotorasib was made in Nov. 2017, and by Aug. 2018, Phase 1/2 clinical studies had begun. The FDA granted Breakthrough Therapy designation for investigational sotorasib on Dec. 7, 2020.
The analysis of the current competitive landscape and future development of target KRAS G12C reveals that multiple companies, including Mirati Therapeutics, Inc., Amgen, Inc., Eli Lilly & Co., Novartis AG, and Zai Lab Ltd., are growing rapidly in the pharmaceutical industry. These companies have made significant progress in the R&D of drugs targeting KRAS G12C, with various phases of development.
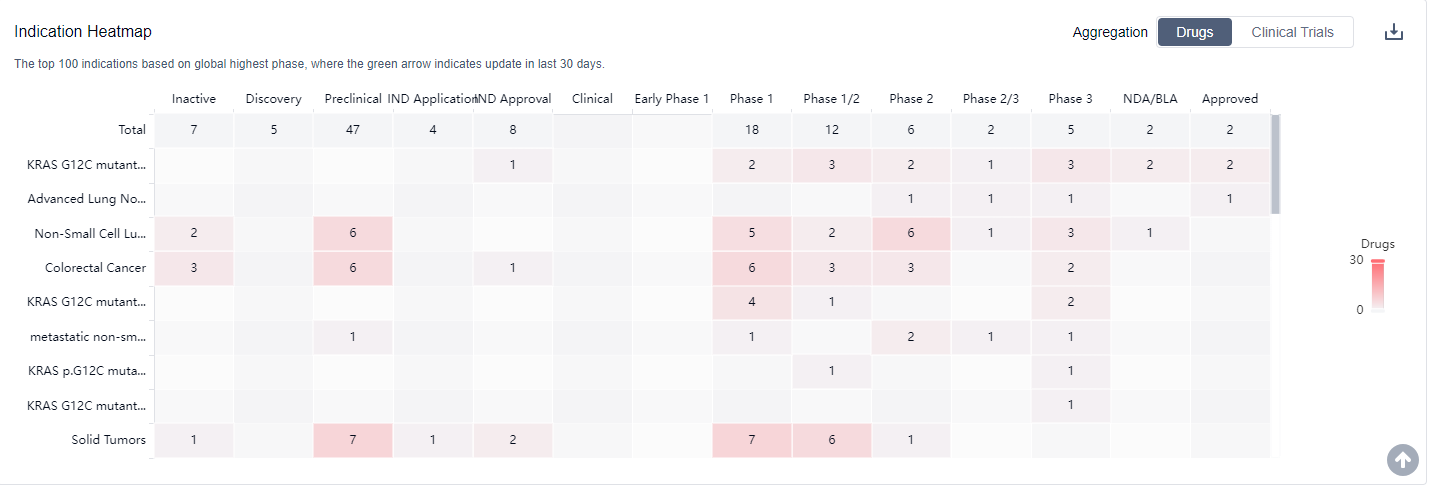
Drugs targeting KRAS G12C have been approved for indications such as KRAS G12C mutant Non-small Cell Lung Cancer, Advanced Lung Non-Small Cell Carcinoma, Colorectal Cancer, and metastatic non-small cell lung cancer. The highest phase of development for indications related to KRAS G12C is Phase 3.
Small molecule drugs are progressing most rapidly under the current target, indicating intense competition around the innovative drugs. Biosimilars, such as PROTACs and Oligonucleotide, are also ranking high, further contributing to the competitive landscape.
Overall, the target KRAS G12C presents a competitive landscape with multiple companies, various indications, and different drug types. The future development of drugs targeting KRAS G12C holds great potential, especially in countries like China, where significant progress has been made.
How do they work?
KRAS G12C inhibitors are a type of targeted therapy used in biomedicine to treat certain types of cancer. KRAS is a gene that plays a critical role in cell signaling and growth regulation. However, mutations in the KRAS gene, such as the G12C mutation, can lead to uncontrolled cell growth and the development of cancer.
KRAS G12C inhibitors specifically target the G12C mutation of the KRAS gene. These inhibitors work by blocking the activity of the mutated KRAS protein, which is essential for cancer cell survival and proliferation. By inhibiting the function of the mutated protein, KRAS G12C inhibitors can help slow down or stop the growth of cancer cells.
These inhibitors are considered a promising therapeutic approach, particularly in the treatment of non-small cell lung cancer (NSCLC) that carries the KRAS G12C mutation. They are designed to specifically target the mutated protein while sparing normal, healthy cells, thereby minimizing side effects.
It's important to note that KRAS G12C inhibitors are still under investigation and clinical trials are being conducted to evaluate their safety and effectiveness. They represent a significant advancement in targeted cancer therapy and hold potential for improving outcomes in patients with KRAS G12C-mutated cancers.
What are KRAS G12C inhibitors used for?
The currently marketed KRAS G12C inhibitors include:
For more information, please click on the image below.
What are KRAS G12C inhibitors used for?
KRAS G12C inhibitors are considered a promising therapeutic approach, particularly in the treatment of non-small cell lung cancer (NSCLC) that carries the KRAS G12C mutation.For more information, please click on the image below to log in and search.
How to obtain the latest development progress of KRAS G12C inhibitors?
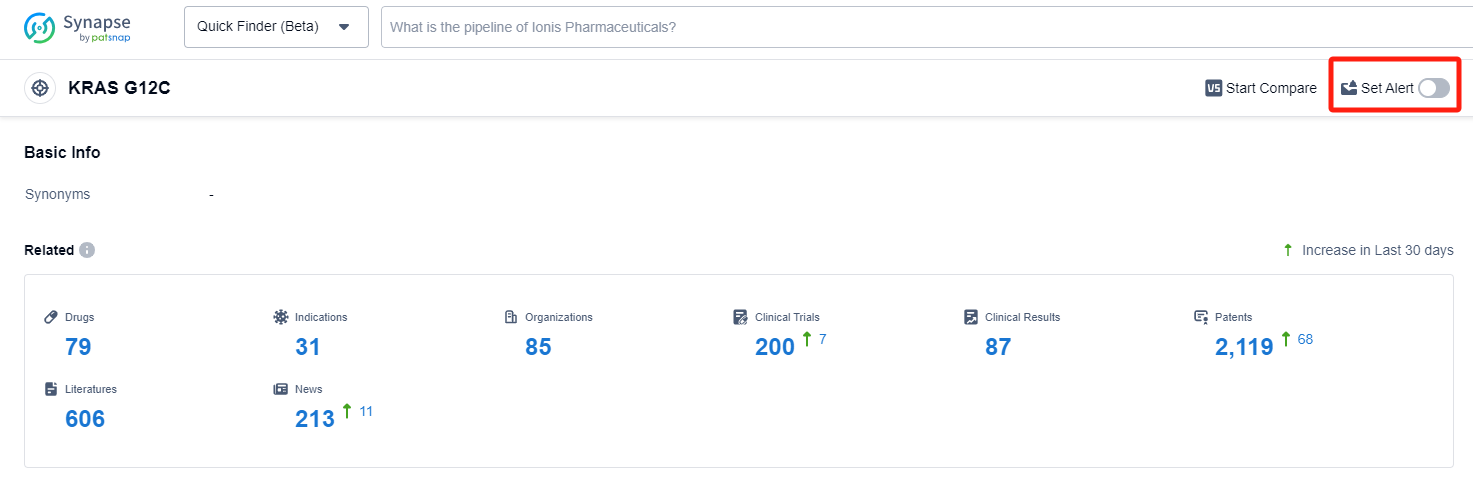
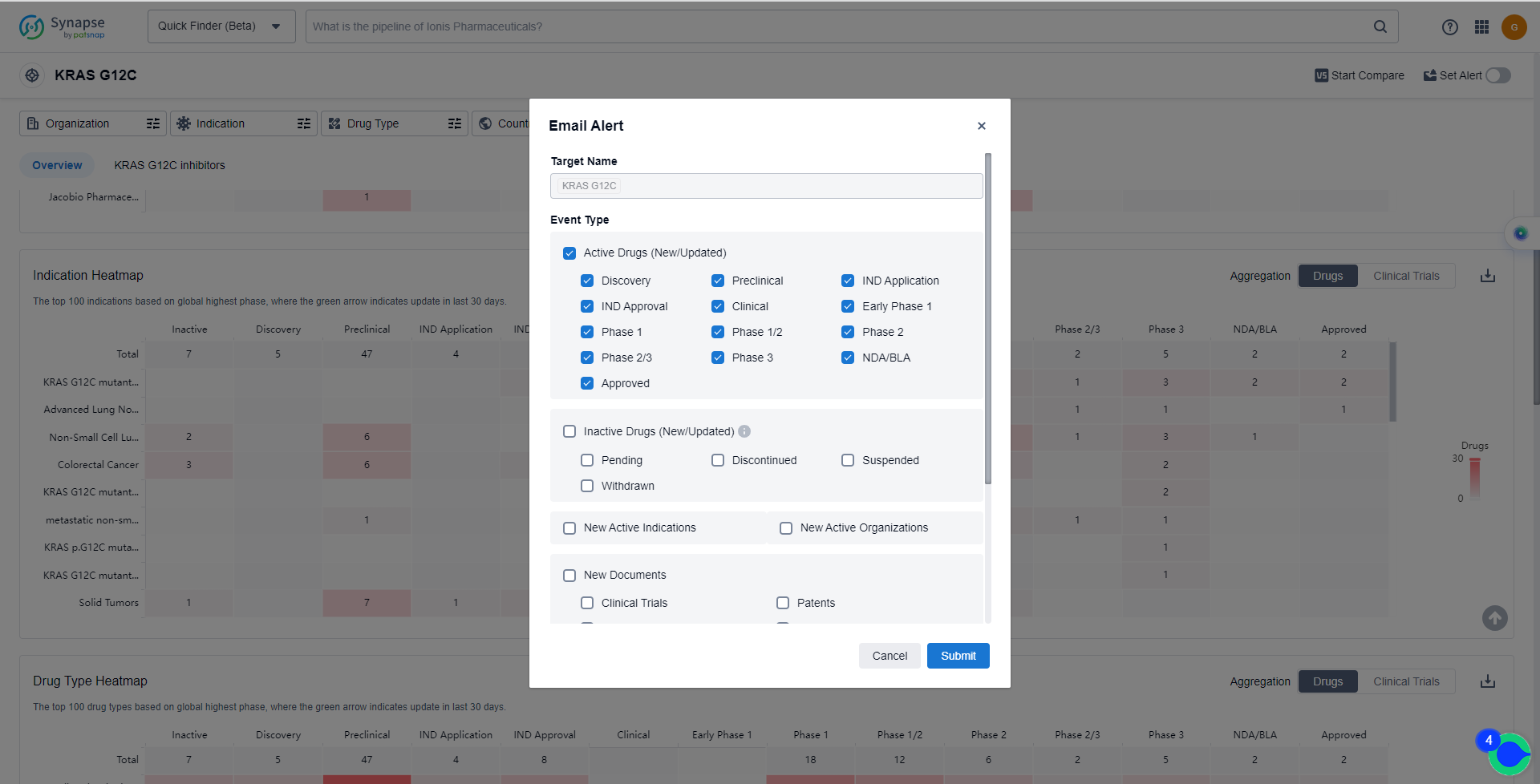
In the Synapse database, you can keep abreast of the latest research and development advances of KRAS G12C inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!