Pharmaceutical Insights: Doripenem's R&D Progress and its Mechanism of Action on Drug Target
Doripenem's R&D Progress
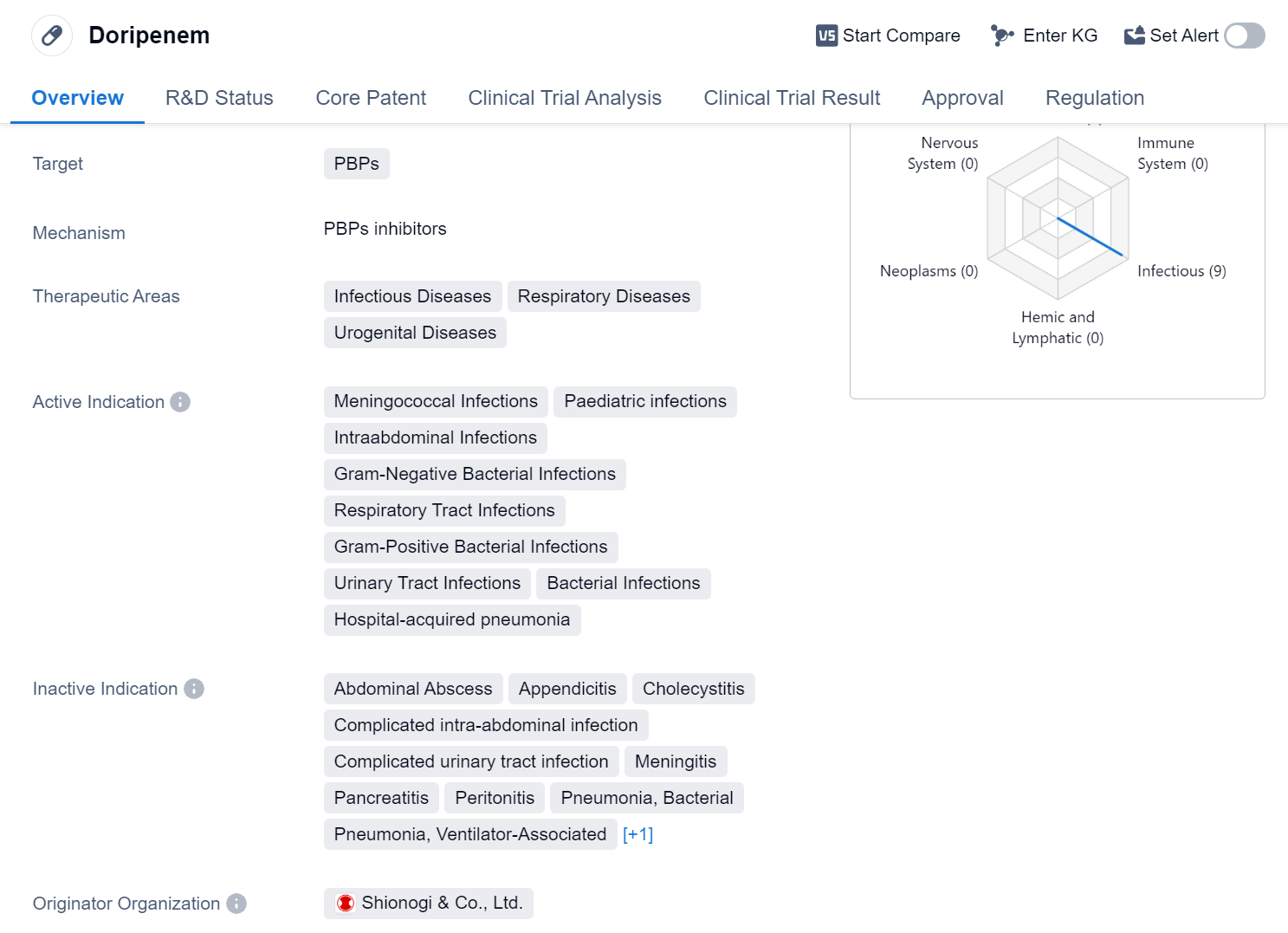
Doripenem is a small molecule drug that belongs to the class of antibiotics known as carbapenems. It primarily targets penicillin-binding proteins (PBPs) to inhibit bacterial cell wall synthesis. This drug has shown efficacy in treating a range of infectious diseases, respiratory diseases, and urogenital diseases.
In terms of therapeutic areas, Doripenem has been approved for the treatment of various conditions including meningococcal infections, pediatric infections, intraabdominal infections, gram-negative bacterial infections, respiratory tract infections, gram-positive bacterial infections, urinary tract infections, bacterial infections, and hospital-acquired pneumonia. This broad spectrum of indications highlights the versatility of Doripenem in combating different types of bacterial infections.
Doripenem was developed by Shionogi & Co., Ltd., a pharmaceutical company based in Japan. It received its first approval in Japan in July 2005, making it available for use in the Japanese market. The drug has also undergone clinical trials in China, reaching Phase 3, indicating its potential for approval in the Chinese market in the future.
One notable regulatory aspect of Doripenem is its designation as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives and benefits to the manufacturer, such as extended market exclusivity and financial support for research and development.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Doripenem: PBPs inhibitors
PBPs inhibitors are a class of drugs that target penicillin-binding proteins (PBPs). PBPs are enzymes found in the cell walls of bacteria and are involved in the synthesis and maintenance of the bacterial cell wall. These enzymes catalyze the cross-linking of peptidoglycan chains, which provides structural integrity to the cell wall.
PBPs inhibitors work by binding to the active site of PBPs and inhibiting their enzymatic activity. This disruption in cell wall synthesis weakens the bacterial cell wall, making it more susceptible to damage and ultimately leading to bacterial cell death. By targeting PBPs, these inhibitors effectively hinder bacterial growth and replication.
In the field of biomedicine, PBPs inhibitors are commonly used as antibiotics to treat bacterial infections. They are particularly effective against Gram-positive bacteria, as these organisms have a thicker peptidoglycan layer in their cell walls, making them more reliant on PBPs for cell wall synthesis. By inhibiting PBPs, these drugs can selectively target and eliminate Gram-positive bacteria while having minimal impact on human cells, which lack peptidoglycan in their cell walls.
Examples of PBPs inhibitors include penicillin, cephalosporins, and carbapenems. These drugs have been widely used for decades and have played a crucial role in treating various bacterial infections. However, the emergence of antibiotic resistance has posed a significant challenge in recent years, limiting the effectiveness of PBPs inhibitors.
Drug Target R&D Trends for Doripenem
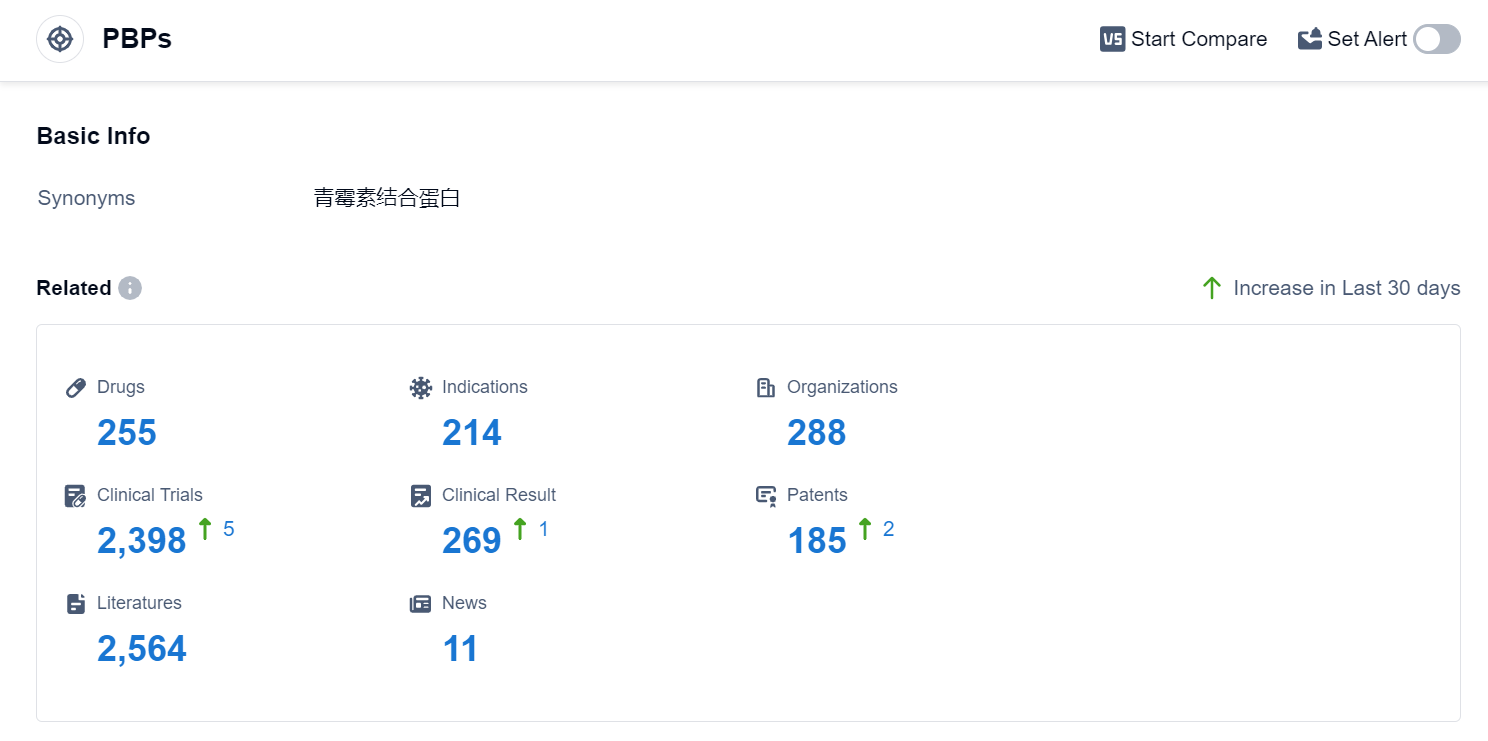
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 255 PBPs drugs worldwide, from 288 organizations, covering 214 indications, and conducting 2398 clinical trials.
The analysis of target PBPs in the pharmaceutical industry reveals that Pfizer Inc., Meiji Holdings Co., Ltd., Takeda Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., and Merck & Co., Inc. are the companies growing fastest under this target. Bacterial infections, infectious diseases, and urinary tract infections are the most common indications for drugs under the target PBPs. Small molecule drugs and synthetic peptides are the drug types progressing most rapidly. China, Japan, and the United States are the countries/locations developing fastest under the target PBPs, with China showing significant progress. The current competitive landscape suggests intense competition and ongoing research and development efforts in the field of target PBPs. The future development of target PBPs is likely to be influenced by the advancements in these companies, indications, drug types, and countries/locations.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Doripenem is a small molecule drug developed by Shionogi & Co., Ltd. that targets PBPs to combat a wide range of infectious diseases, respiratory diseases, and urogenital diseases. With its approval in Japan and ongoing Phase 3 trials in China, Doripenem has the potential to provide effective treatment options for patients in need. Its designation as an orphan drug further highlights its importance in addressing rare diseases and conditions.