Freeline Conveys Clinical Findings from Phase 1/2 Trial for FLT201 in Gaucher Disease
Freeline Therapeutics Holdings plc revealed optimistic preliminary results concerning safety, tolerability, and enzyme action from the ongoing Phase 1/2 GALILEO-1 study. This trial evaluates FLT201, their adeno-associated virus gene therapy contender, applicable to Gaucher disease.
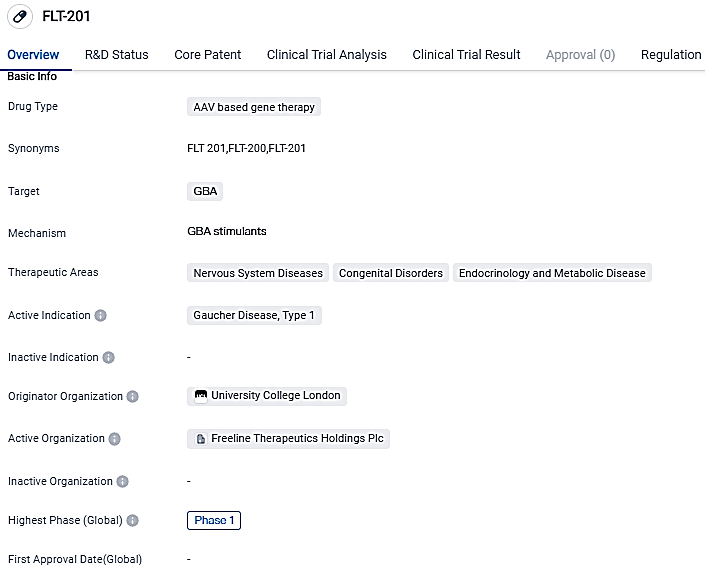
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Gaucher disease is a damaging genetic ailment caused by a lack of the GCase enzyme, leading to a harmful substance accumulation, resulting in enlarged spleen and liver, anemia, bone discomfort, and diminished lung function.
Preliminary findings from the first two patients in the GALILEO–1 study demonstrated a safe and tolerable reaction, with a single dosage of FLT201 resulting in an explosive multiplication in GCase activities in plasma and normalized leukocyte GCase activities.
"Despite significant improvements brought about by existing treatment methods, many patients still suffer from continuous symptoms and the current treatment methods require a rigorous lifelong regimen. Those with Gaucher disease merit superior therapies. FLT201 offers a hopeful new strategy as a one–time experimental gene therapy. With the initial clinical data at hand, I'm enthusiastic about its potential," stated Pilar Giraldo, M.D., Ph.D., a hematologist at the Spanish Foundation for the Study and Therapy of Gaucher Disease.
“At Freeline, our mission is to fully realize the capability of gene therapy by enhancing every aspect of our candidate products. FLT201 embodies this philosophy. It employs our exclusive capsid to allow high expression at minimal dosages and our unique GCase variant to challenge the brief lifespan of the typical GCase," declared Michael Parini, CEO of Freeline.
Michael Parini added, "Our preclinical results for FLT201 record sturdy enhancements in plasma GCase, effectively clearing the detrimental substrate than the prevailing standard-of-care and thus, taken up by the tissue affected by the ailment. These clinical findings affirm our preclinical data are now translating, and we pledge to promptly mobilize FLT201."
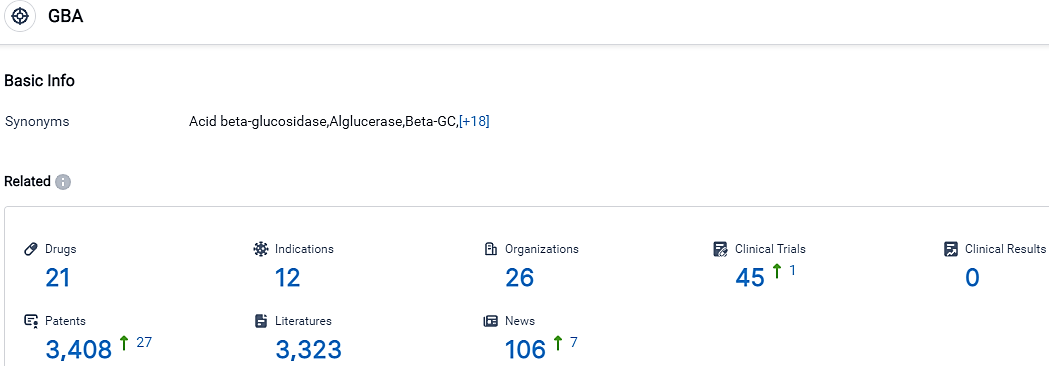
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 14, 2023, there are 21 investigational drugs for the GBA target, including 12 indications, 26 R&D institutions involved, with related clinical trials reaching 45,and as many as 3408 patents.
FLT201 is designed to generate durable increases in glucocerebrosidase and reduce the accumulation of harmful substrates, with the aim of providing a one-time treatment that can stop disease progression, improve outcomes, and free people from lifelong treatment. Further research and development will be needed to determine the safety and efficacy of FLT-201 in treating Gaucher Disease.