Nature releases preclinical outcomes for ABBV-CLS-484, a PTPN2/N1 inhibitor in Cancer Immunotherapy
Nature published an announcement from AbbVie, the Broad Institute of MIT and Harvard, and Calico Life Sciences. This announcement outlines their discovery and preclinical findings, showing that the experimental drug ABBV-CLS-484 holds potential as a pioneering PTPN2/N1 phosphatase inhibitor that could be administered orally and bolster anti-cancer immunity.
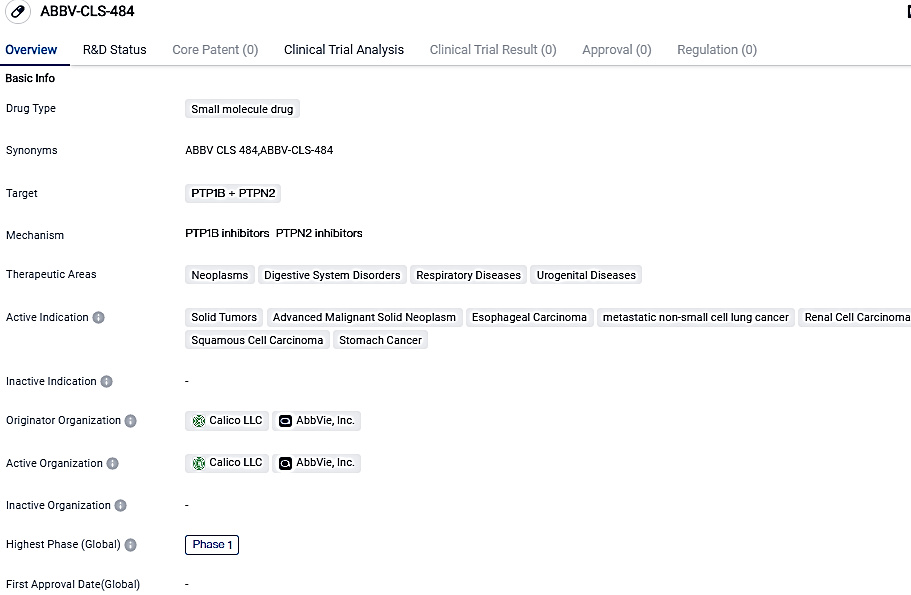
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
According to research carried out by AbbVie in alliance with the Broad Institute and Calico, the development of ABBV-CLS-484 presents an innovative approach in the field of cancer immunotherapy.
Broad Institute scientists had revealed protein tyrosine phosphatase non-receptor type 2 along with its close relative PTPN1 as potential targets for cancer immunotherapy using an in vivo CRISPR screen. However, this complicated target class was previously categorized as "undruggable". The AbbVie team managed to bypass these obstacles and developed the dual PTPN2/N1 inhibitor, ABBV-CLS-484, through an intensive drug design process and refinement of drug-like properties.
The discovery team at AbbVie, the Broad, and Calico dug further into the biological processes and way of operation of ABBV-CLS-484. Preliminary findings unveiled that ABBV-CLS-484 therapy enhances the tumor's inherent reaction to interferon and heightens the activation and mechanism of multiple immune cell variants- a boost to cellular pathways such as JAK-STAT signaling in animal models was noticed.
In murine cancer models resilient to PD-1 blockade, single therapy with ABBV-CLS-484 fosters robust enemy-tumor immunity. Using in vivo studies and singular cell transcriptional profiling of tumor-penetrating lymphocytes from ABBV-CLS-484 treated mice, the researchers demonstrated that ABBV-CLS-484 inflames the tumor environment encouraging NK and CD8+ T-cell functioning.
"Immunotherapies like PD-1 blockade have revolutionized cancer treatment; however, many patients do not respond well to them, and a significant unmet need remains," said Christina Baumgartner, Ph.D., co-first author and senior principal research scientist, AbbVie. "Through the extraordinary efforts of AbbVie's medicinal chemistry team to drug the undruggable, we now have a potential first-in-class PTPN2/N1 inhibitor. We're excited to share its biology and mechanism of action, and look forward to further evaluating it in the clinic."
"With the exceptional efforts of the AbbVie medicinal chemistry team to drug the 'undruggable', we now have a first-in-the-league PTPN2/N1 inhibitor. We are eager to disclose its biological processes and operational mechanism, and we anticipate further clinical evaluation." expressed Christina Baumgartner, Ph.D., co-leading author and elite research scientist at AbbVie.
"This offers a unprecedented chance to examine how immune responses function," claimed Robert Manguso, Ph.D., co-senior author of the study, associate member at the Broad, and Assistant Professor at the Massachusetts General Hospital Center for Cancer Research and Harvard Medical School. "The scope of manipulating this signaling pathway further in medical studies carries considerable importance."
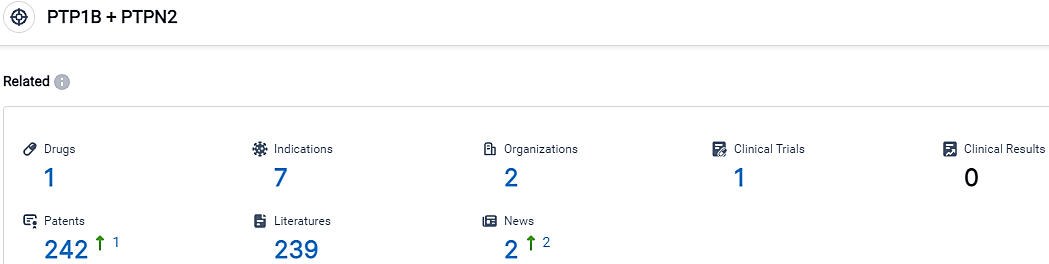
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 14, 2023, there are 1 investigational drugs for the PTP1B/PTPN2 target, including 7 indications, 2 R&D institutions involved, with related clinical trials reaching 1,and as many as 242 patents.
ABBV-CLS-484 is the first active-site phosphatase inhibitor to enter clinical evaluation as a cancer therapy and is currently in an AbbVie and Calico-led Phase 1 clinical trial in solid tumors. This drug holds promise for the future treatment of solid tumors, advanced malignant solid neoplasms, esophageal carcinoma, metastatic non-small cell lung cancer, renal cell carcinoma, squamous cell carcinoma, and stomach cancer.