Unleashing the Power of Fremanezumab-VFRM: A Comprehensive Review on R&D Breakthroughs
Fremanezumab-VFRM's R&D Progress
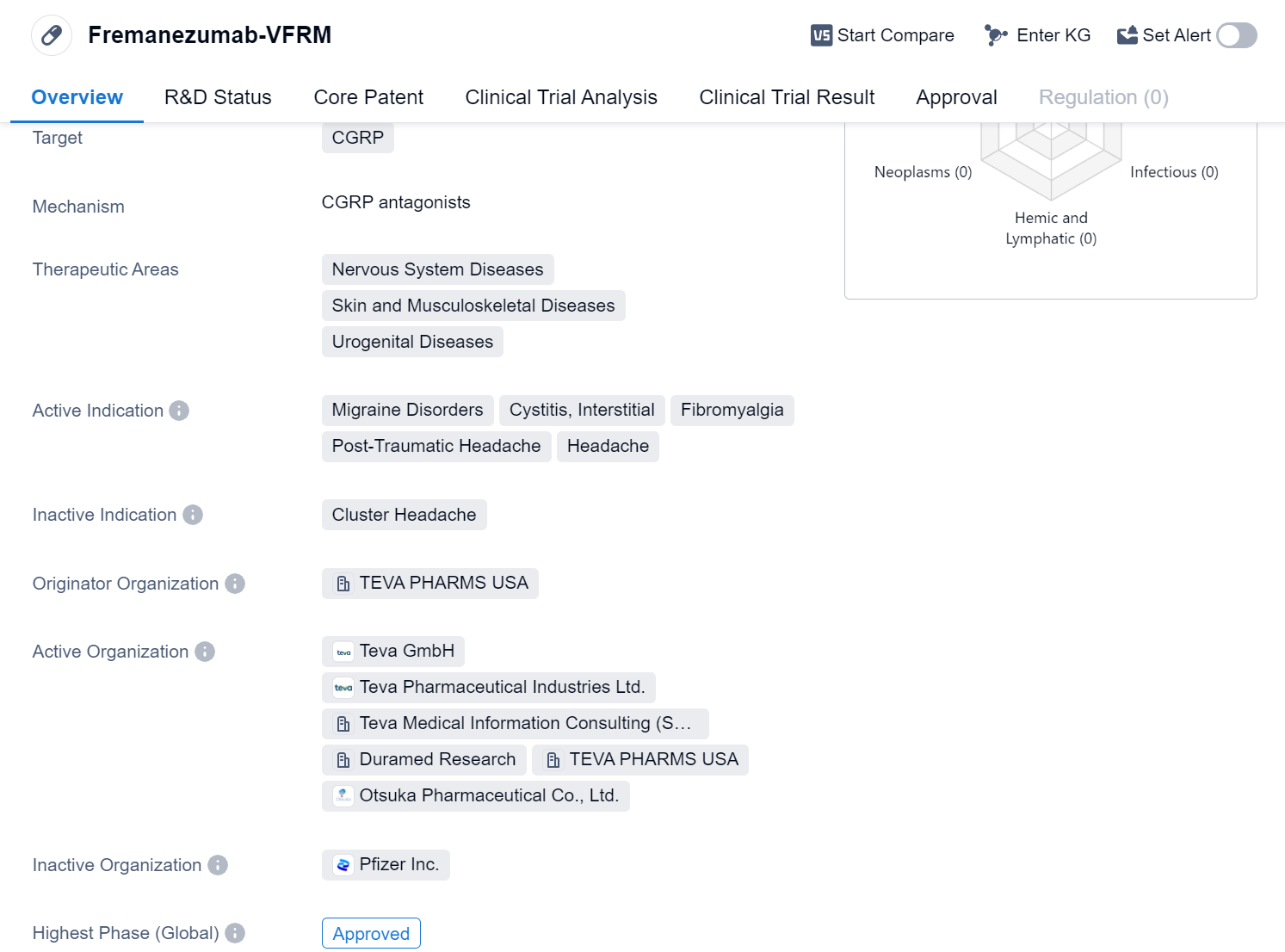
Fremanezumab-VFRM is a monoclonal antibody drug that targets the calcitonin gene-related peptide (CGRP). It is primarily used in the treatment of various nervous system diseases, skin and musculoskeletal diseases, and urogenital diseases. The drug has been approved for use in the United States and is indicated for the treatment of migraine disorders, cystitis, interstitial, fibromyalgia, post-traumatic headache, and headache.
Fremanezumab-VFRM is developed by TEVA PHARMS USA, a pharmaceutical company specializing in the production of generic drugs and innovative treatments. The drug has reached the highest phase of development, which is the approved stage, in the global market.
In China, Fremanezumab-VFRM is currently in phase 3 of development. This phase is crucial in determining whether the drug will receive approval for use in China.
The first approval of Fremanezumab-VFRM took place in September 2018 in the United States. This signifies that it was the first country to grant regulatory approval for the drug. The approval process involves rigorous evaluation of the drug's clinical data, safety profile, and manufacturing processes to ensure its quality and efficacy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Fremanezumab-VFRM: CGRP antagonists
CGRP antagonists are a class of drugs that work by blocking the action of calcitonin gene-related peptide (CGRP), a neuropeptide involved in the transmission of pain signals. From a biomedical perspective, CGRP antagonists are primarily used in the treatment of migraine headaches. CGRP is known to play a significant role in the pathophysiology of migraines, and by inhibiting its action, these drugs can help prevent or reduce the frequency and severity of migraine attacks.
CGRP antagonists are designed to bind to CGRP receptors, preventing CGRP from binding and activating these receptors. This inhibition of CGRP activity leads to a reduction in the dilation of blood vessels and the release of inflammatory substances, both of which are implicated in migraine attacks. By blocking CGRP, these drugs help to alleviate the pain and other symptoms associated with migraines.
It's important to note that CGRP antagonists are a relatively new class of drugs specifically developed for the treatment of migraines. They are typically administered as oral tablets or as injectable formulations. CGRP antagonists have shown promising results in clinical trials and have been approved by regulatory authorities for the prevention of migraines in certain patient populations. However, like any medication, CGRP antagonists may have potential side effects and should be used under the guidance of a healthcare professional.
Drug Target R&D Trends for Fremanezumab-VFRM
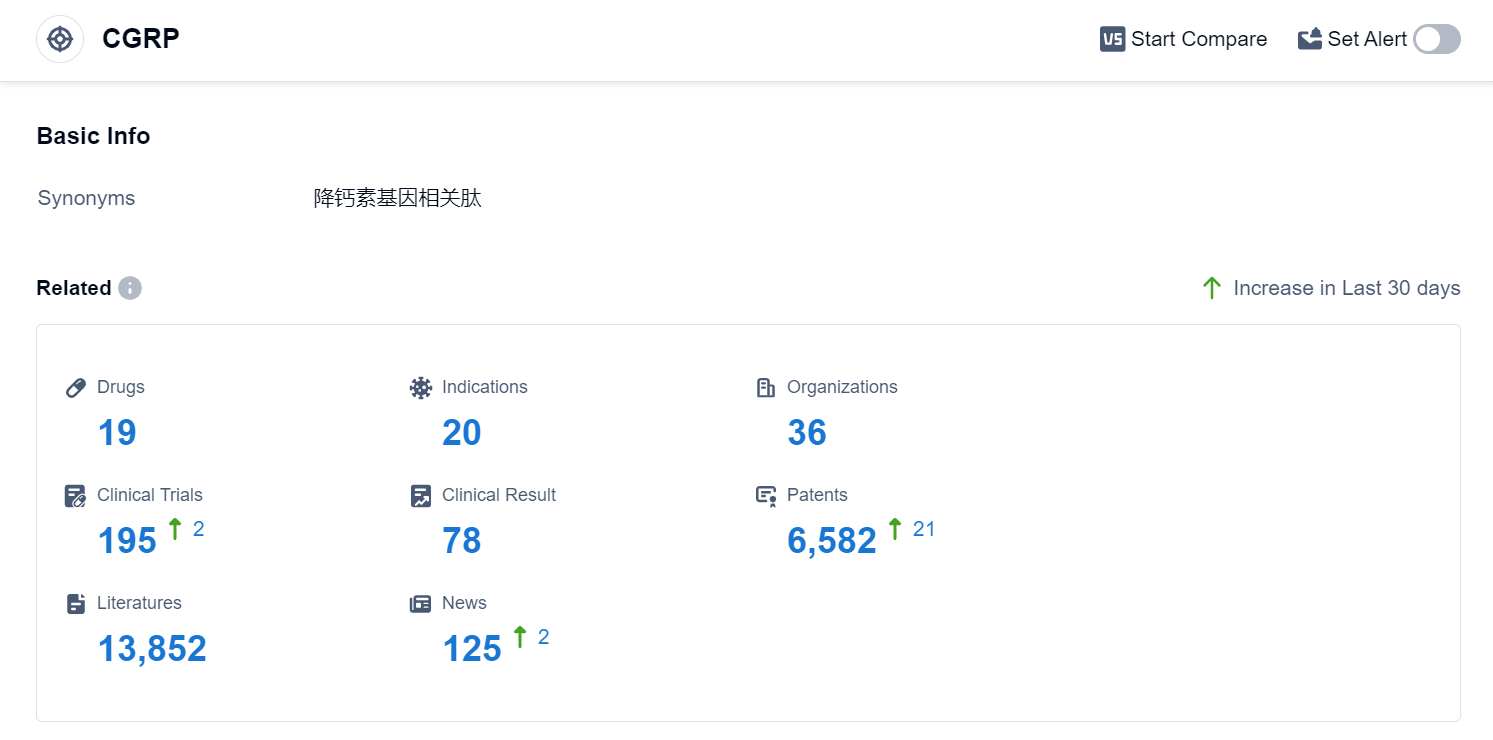
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 19 CGRP drugs worldwide, from 36 organizations, covering 20 indications, and conducting 195 clinical trials.
The analysis of the current competitive landscape for target CGRP reveals that companies like Eli Lilly & Co., Lundbeck Foundation, Pfizer Inc., Teva Pharmaceutical Industries Ltd., and AbbVie, Inc. are leading the way in terms of R&D progress. These companies have drugs in various stages of development, with Eli Lilly & Co. and Lundbeck Foundation having the highest stage of development. The main indication for drugs targeting CGRP is Migraine Disorders, with several drugs approved for this indication. Monoclonal antibodies and Small molecule drugs are the most rapidly progressing drug types under the current target. The development of biosimilars indicates intense competition in the market. The United States, European Union, and other countries/locations like China, South Korea, and Russia are actively involved in the development of drugs targeting CGRP. The future development of target CGRP holds promise for the treatment of various indications and the potential for market growth in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Fremanezumab-VFRM is a monoclonal antibody drug that targets CGRP and is used in the treatment of various nervous system diseases, skin and musculoskeletal diseases, and urogenital diseases. It has received approval in the United States and is indicated for the treatment of migraine disorders, cystitis, interstitial, fibromyalgia, post-traumatic headache, and headache. The drug is developed by TEVA PHARMS USA and has reached the highest phase of development globally. In China, it is currently in phase 3 of development, indicating ongoing clinical trials. The first approval of Fremanezumab-VFRM occurred in September 2018 in the United States.