Fulcrum Therapeutics and Sanofi Partner on Losmapimod for FSHD Treatment
Fulcrum Therapeutics, Inc., a biopharmaceutical company at the clinical stage that specializes in creating small molecules aimed at enhancing the lives of individuals with genetically defined rare diseases, has revealed a collaboration and licensing agreement with Sanofi. This partnership focuses on the development and commercial distribution of losmapimod, an oral small molecule currently under investigation for treating facioscapulohumeral muscular dystrophy. According to the terms of the agreement, Sanofi is granted exclusive rights to commercialize losmapimod outside the United States.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The agreement on collaboration and licensing merges Fulcrum’s specialization in FSHD with Sanofi’s extensive global presence and strong dedication to treating rare diseases. Losmapimod is presently under evaluation in a global Phase 3 clinical trial aimed at treating FSHD, a chronic and progressive genetic disorder affecting muscles, characterized by substantial muscle cell death and fat accumulation in muscle tissue.
Data from ReDUX4, a Phase 2 clinical trial assessing losmapimod for FSHD treatment, showed a reduction in disease progression and enhanced muscle health. Fulcrum anticipates releasing top-line results from REACH, the global Phase 3 clinical trial, in the fourth quarter of 2024. Should the Phase 3 trial results be favorable, Fulcrum and Sanofi plan to file marketing applications in the U.S., Europe, Japan, and various other regions.
"Sanofi is a recognized leader in the development of treatments for rare neuromuscular diseases, making them the perfect partner to optimize losmapimod's global potential outside the U.S.," stated Alex C. Sapir, Fulcrum’s president and CEO. "This agreement aligns seamlessly with our core strategy, enabling Fulcrum to concentrate on U.S. commercialization preparations for losmapimod, while leveraging Sanofi’s outstanding global commercial capabilities and established presence in key international markets."
“This collaboration presents a thrilling opportunity to expand Sanofi’s rare disease portfolio and deliver the first approved FSHD treatment to patients, utilizing the strength and reach of our commercial organization,” said Burcu Eryilmaz, Sanofi’s Global Head of Rare Diseases. “Losmapimod has demonstrated significant clinical benefits, showcasing its potential to modify the disease and meet the high unmet need for a safe and effective treatment to slow disease progression. Committed to offering new hope and treatment options to patients, we are excited to work closely with Fulcrum as losmapimod progresses through its later stages of development.”
According to the agreement, Fulcrum will receive an initial payment of $80.0 million and is eligible for up to an additional $975.0 million in predetermined regulatory and sales milestones, along with tiered escalating royalties starting in the low-teens based on annual net sales of losmapimod outside the U.S. Moreover, Fulcrum and Sanofi will equally share future global development expenses.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
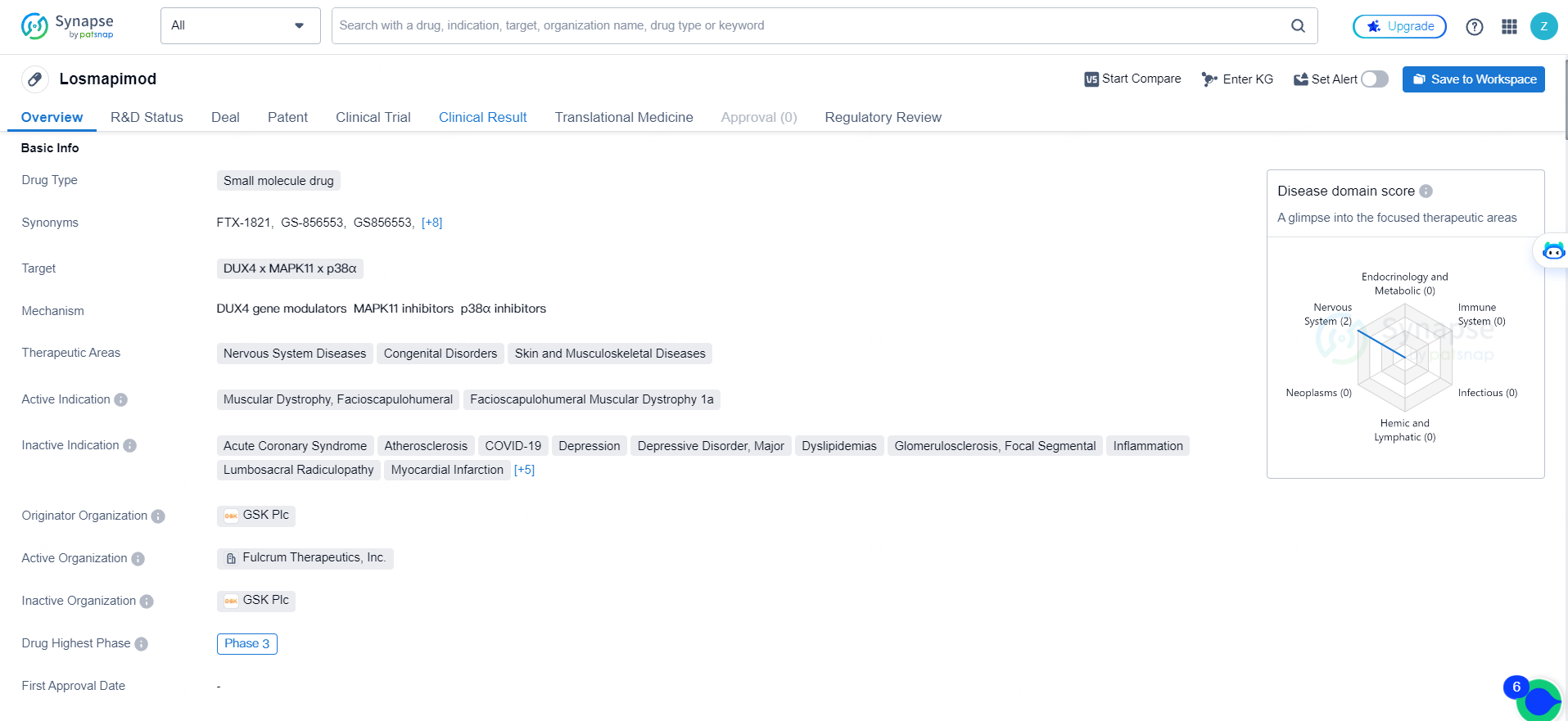
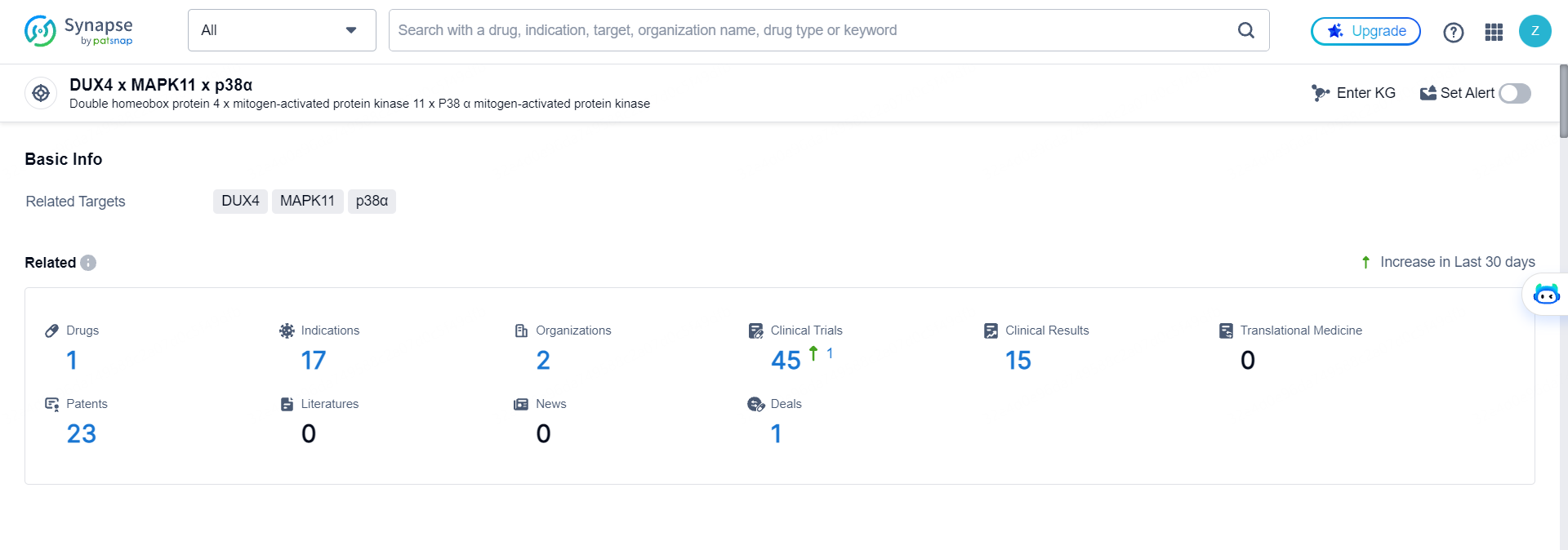
According to the data provided by the Synapse Database, As of May 16, 2024, there are 1 investigational drugs for the DUX4 and MAPK11 and p38α targets, including 17 indications, 2 R&D institutions involved, with related clinical trials reaching 45, and as many as 23 patents.
Losmapimod is a small molecule drug that targets DUX4, MAPK11, and p38α. Losmapimod shows promise in the treatment of muscular dystrophy and related conditions, and its Fast Track and Orphan Drug designations indicate that it may have the potential to address unmet medical needs in these therapeutic areas. As it progresses through clinical trials, Losmapimod has the potential to provide new treatment options for patients with these conditions.