Gaining the Competitive Edge: Effective Strategies for Patent Retrieval and Analysis of ADC Drugs
Kadcyla, a HER2-targeted therapy, received approval and was launched in 2013. It is an antibody-drug conjugate (ADC) used as a single-agent treatment for HER2-positive metastatic breast cancer patients previously treated with trastuzumab and taxane-based chemotherapy, either as monotherapy or in combination. The drug combines trastuzumab, the active component of Herceptin, with DM1, a cytotoxic agent developed by ImmunoGen. DM1 is delivered to HER2-positive breast cancer cells through a stable linker called MCC.
Kadcyla exhibits dual anti-cancer properties: the HER2 inhibition of trastuzumab and the cytotoxicity of DM1. The following outlines the specific steps for patent retrieval of the ADC drug Kadcyla:
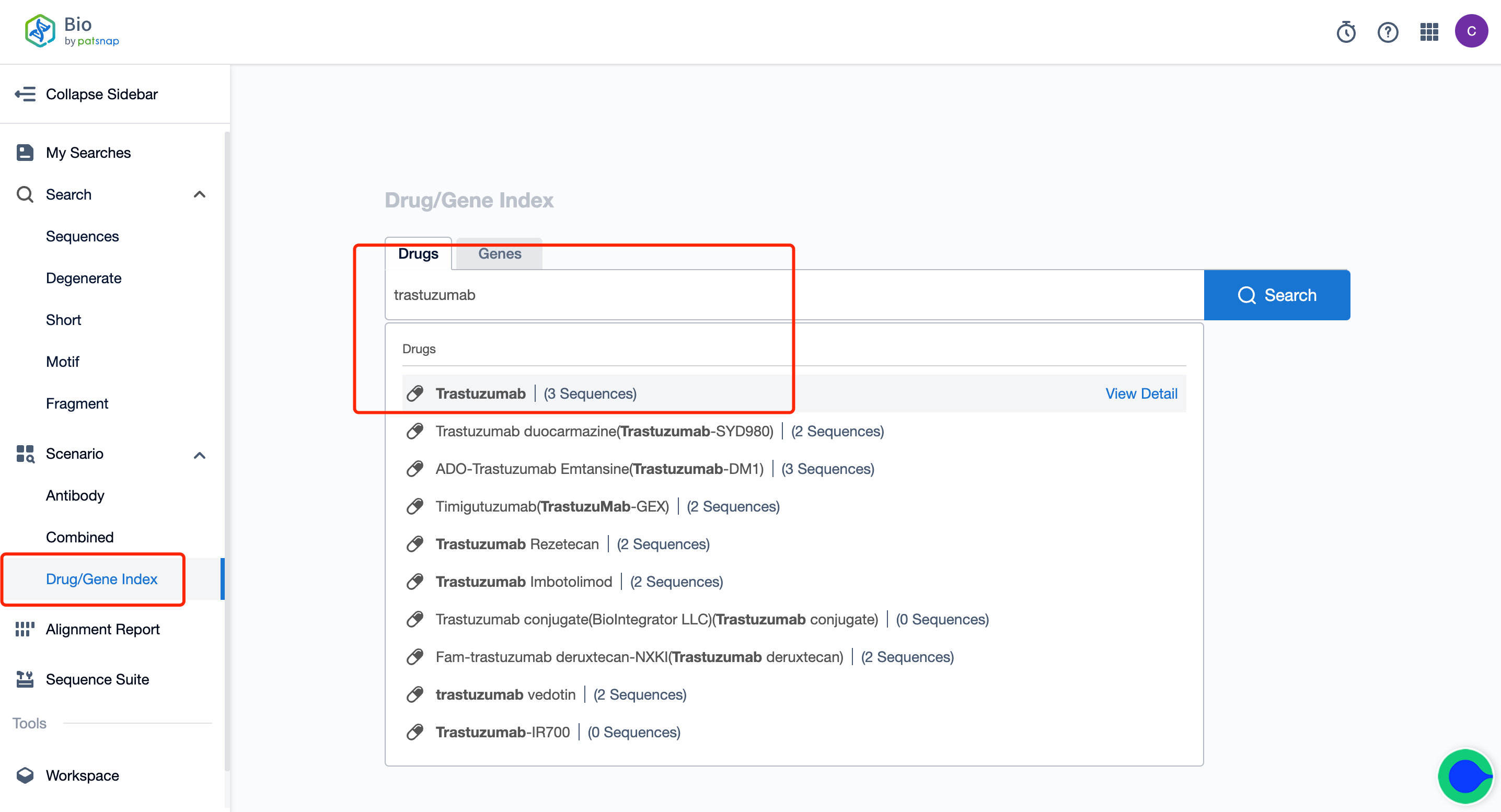
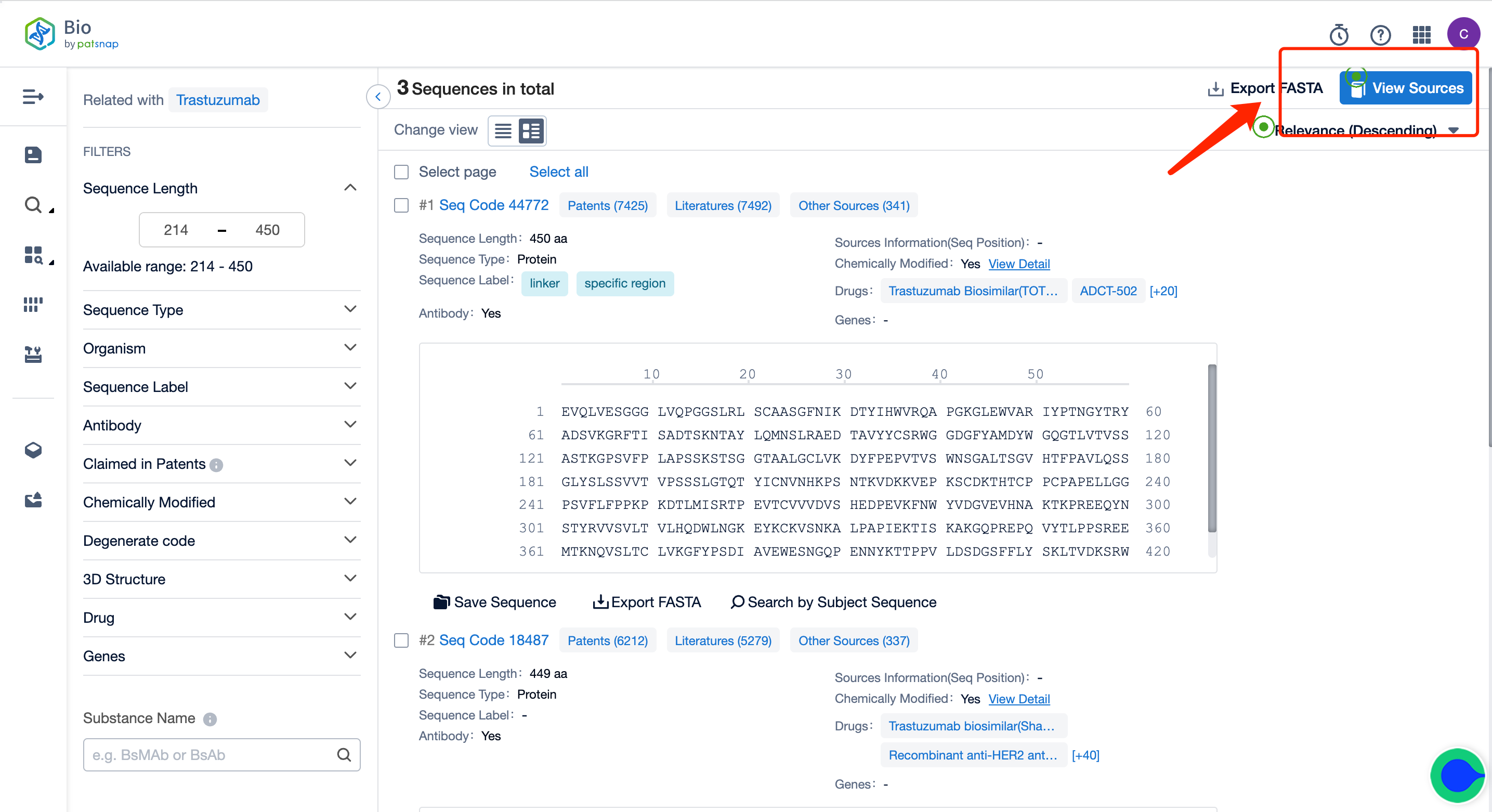
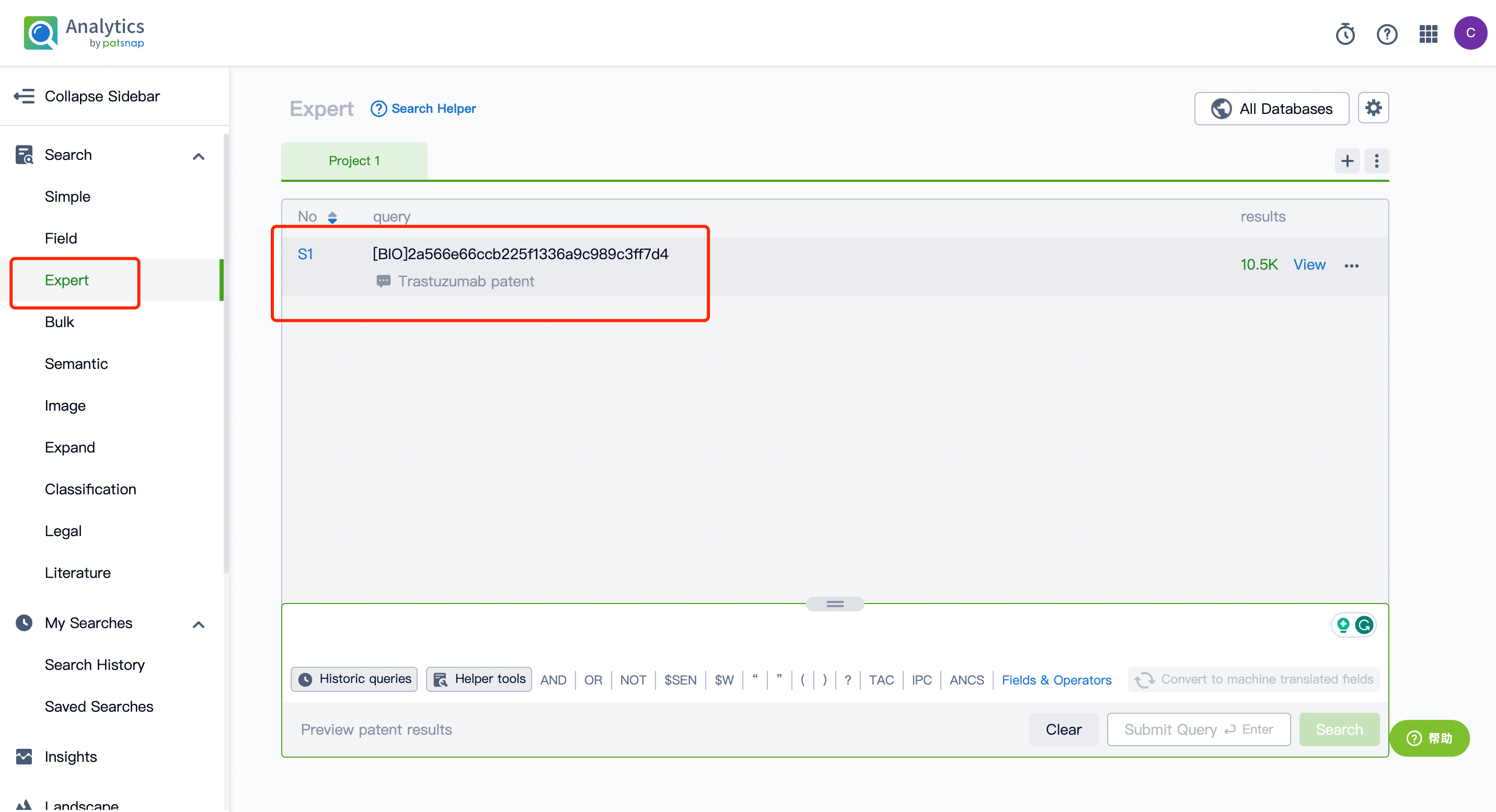
To begin with, register for a free account on the Patsnap Bio Sequence Database; on the database's homepage, enter "Kadcyla trastuzumab" in the drug/gene index search bar to quickly retrieve the sequence details of this antibody.
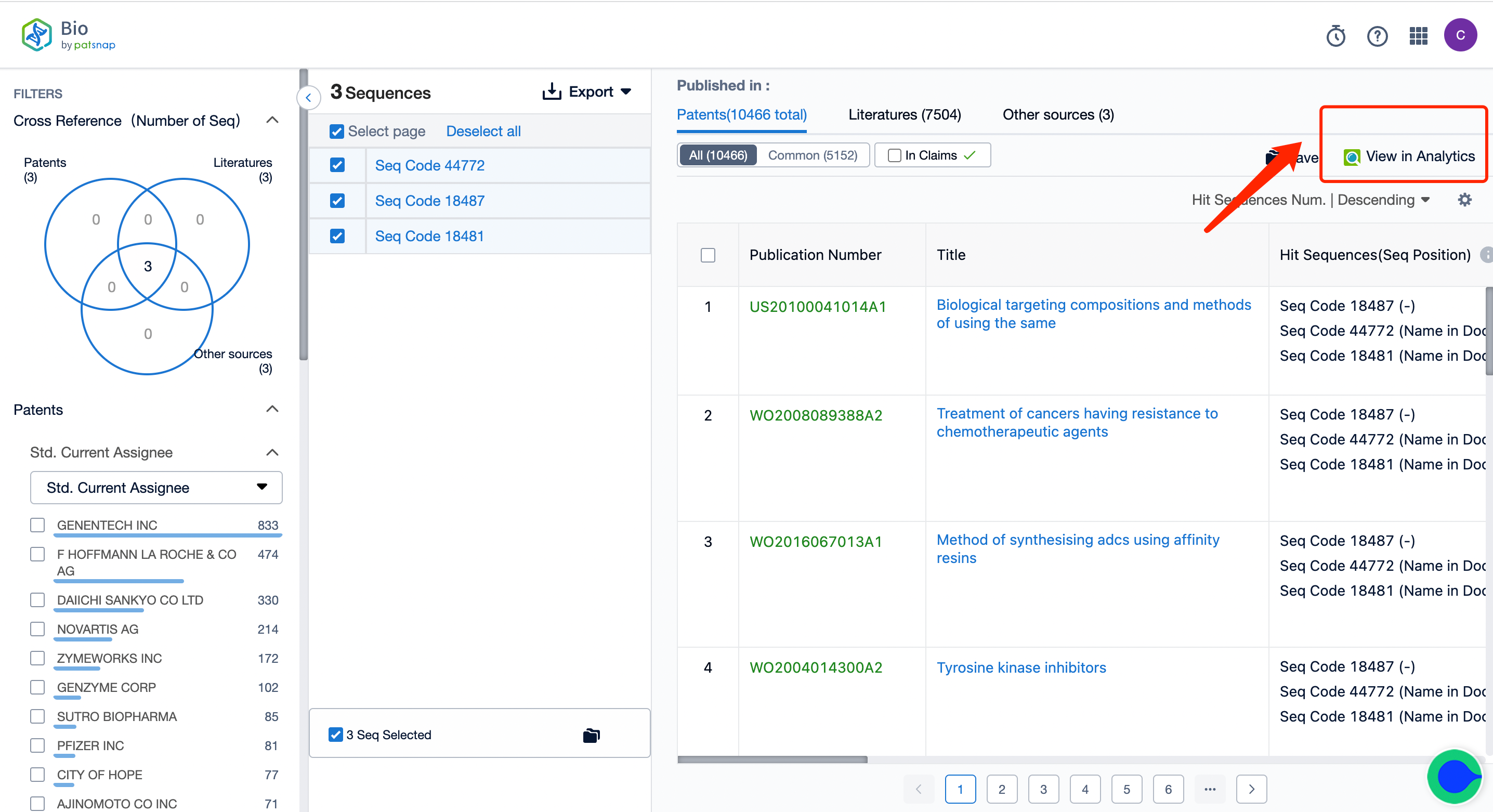
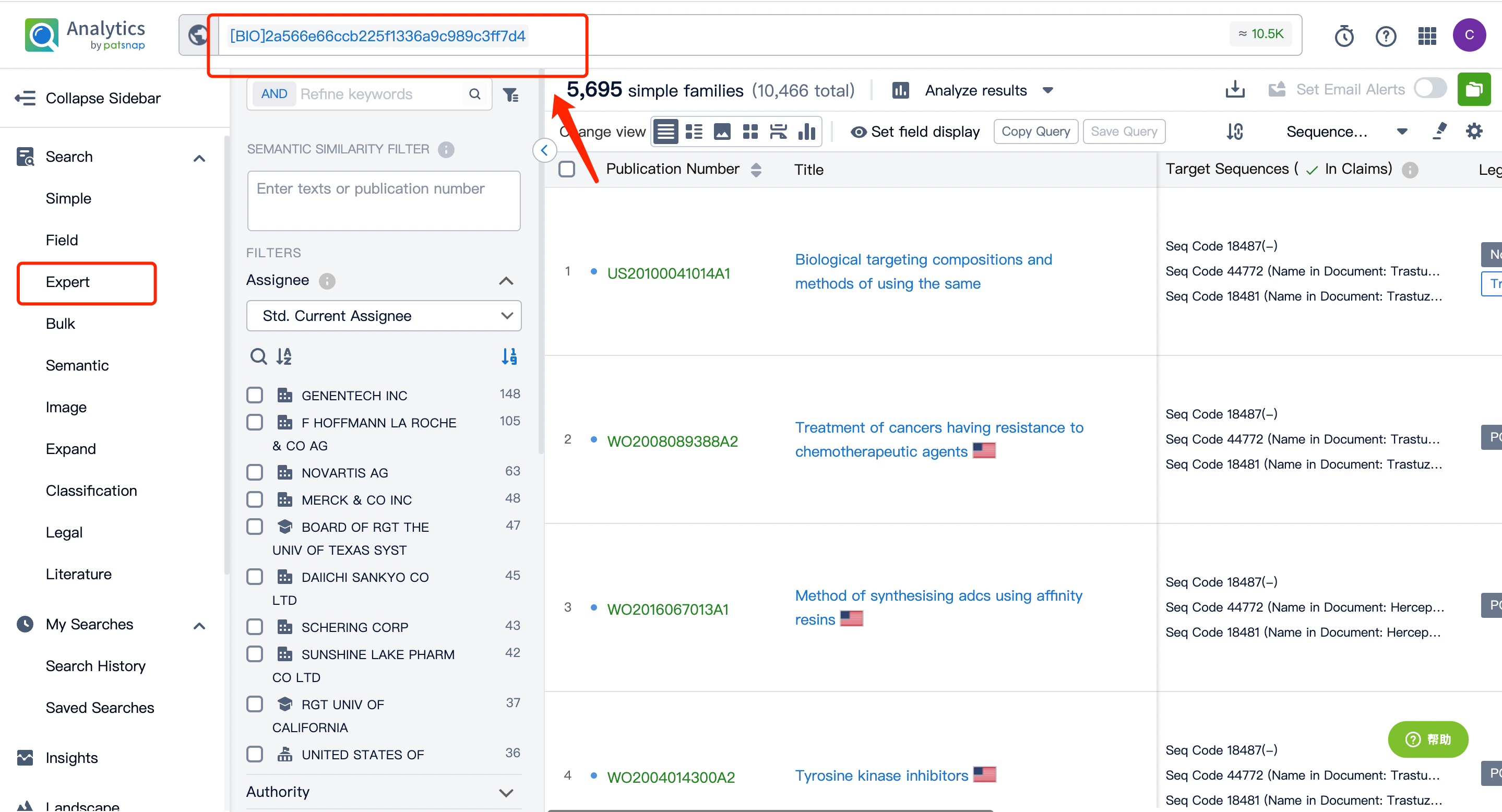
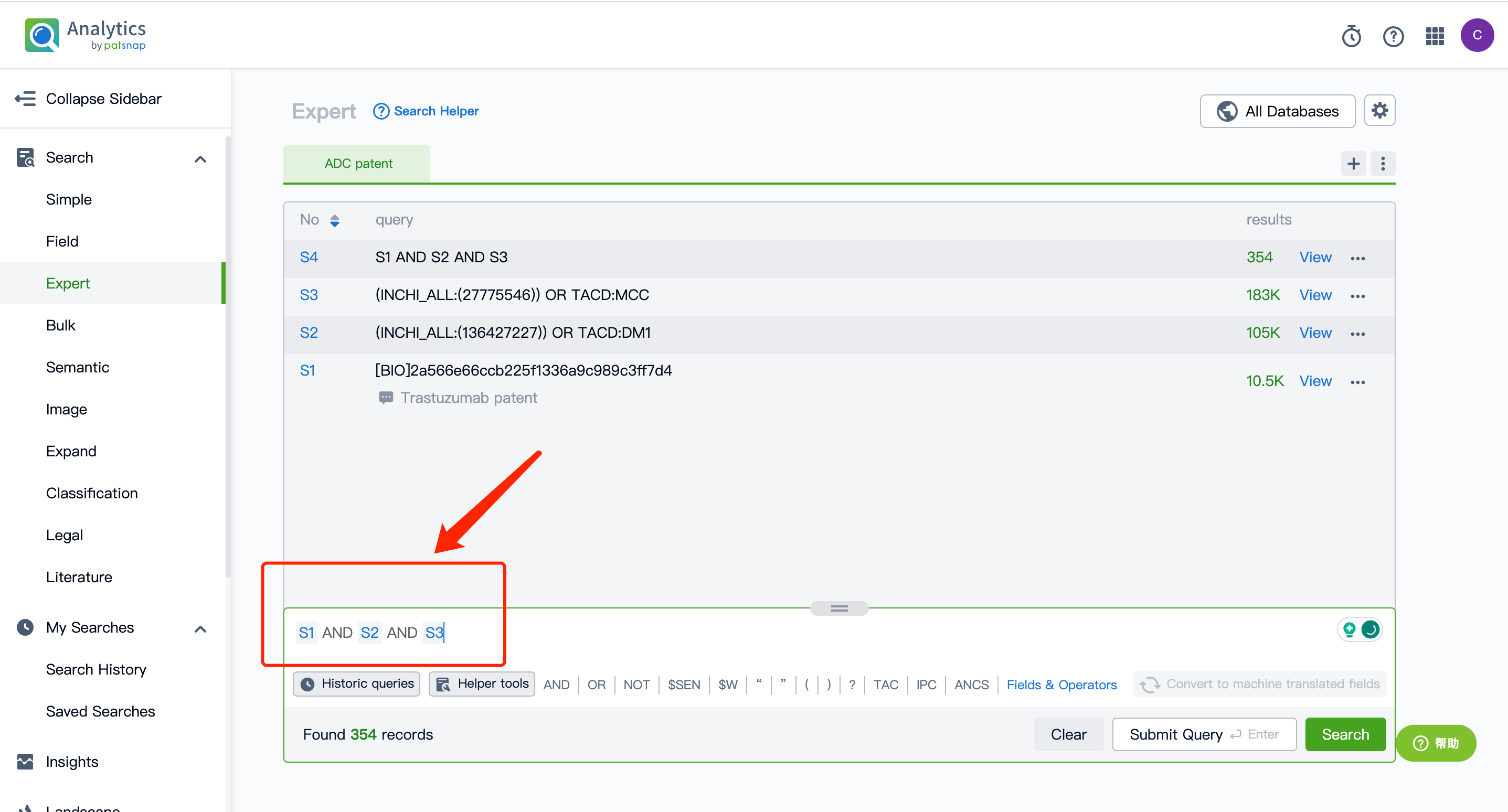
Next, click on the "View in Analytics" option in the upper right corner to access the patent database. You can then generate a search query for the ADC drug Kadcyla's monoclonal antibody Trastuzumab in the search box. Save this retrieval formula within the expert search library.
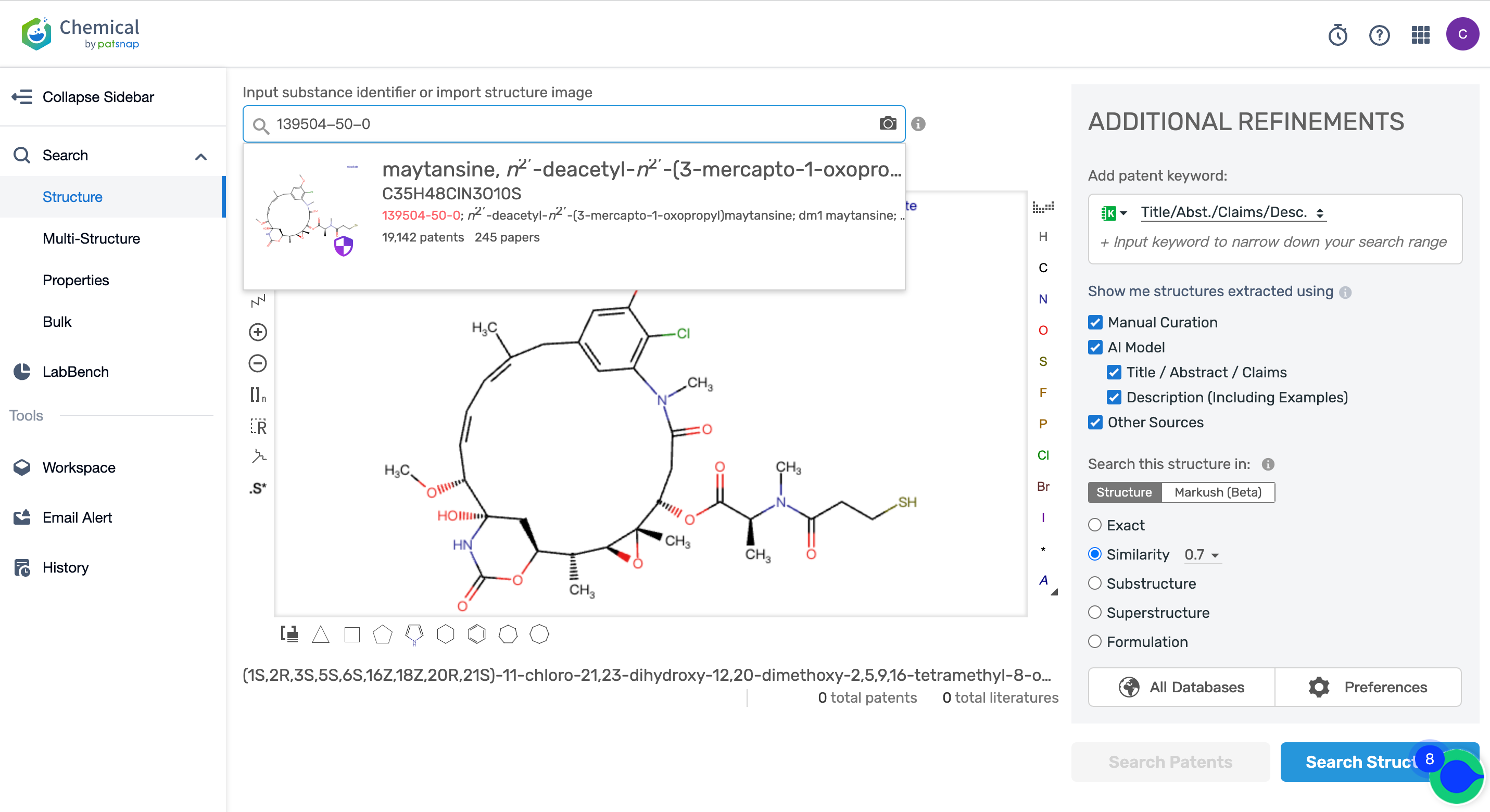
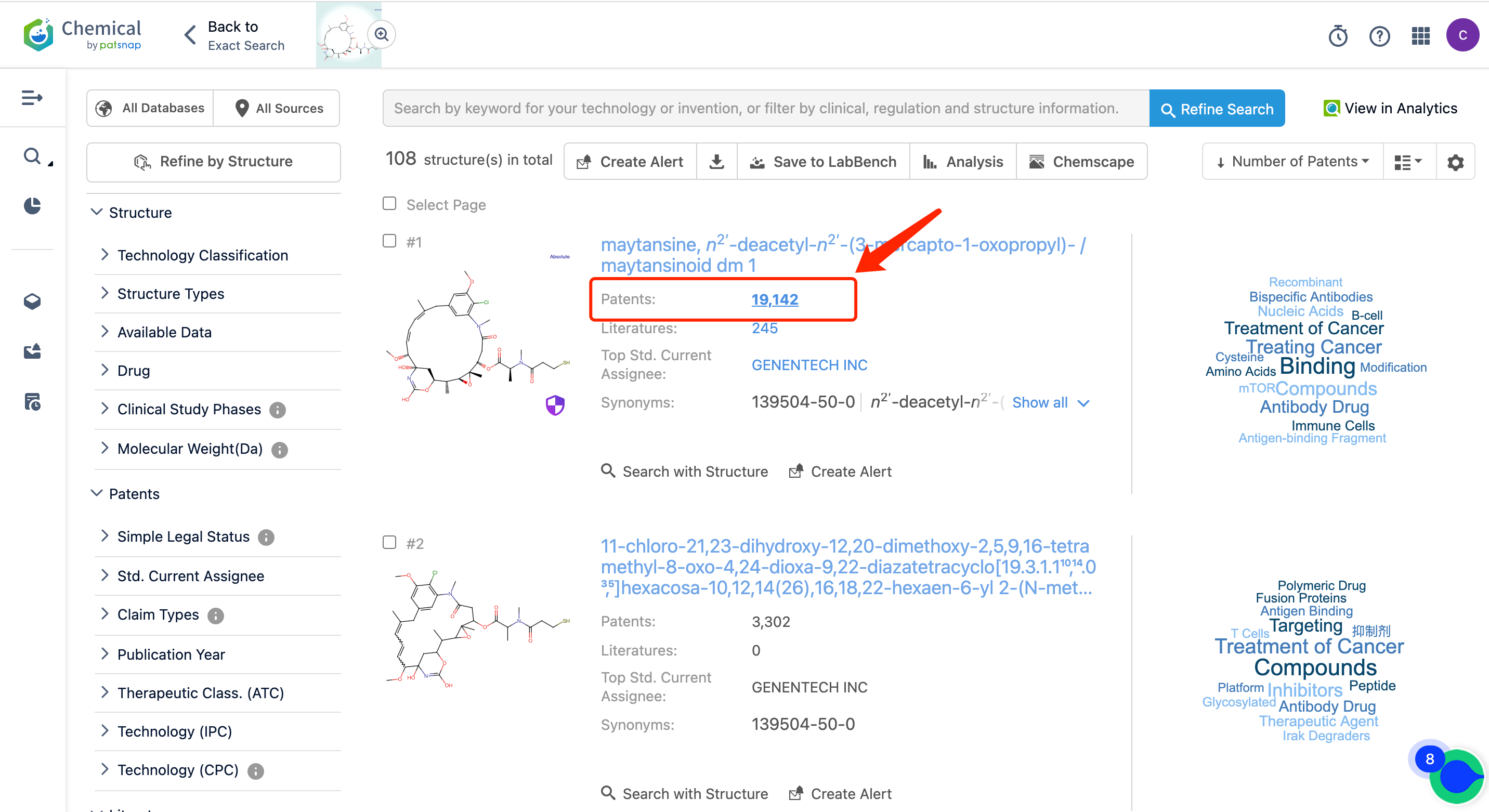
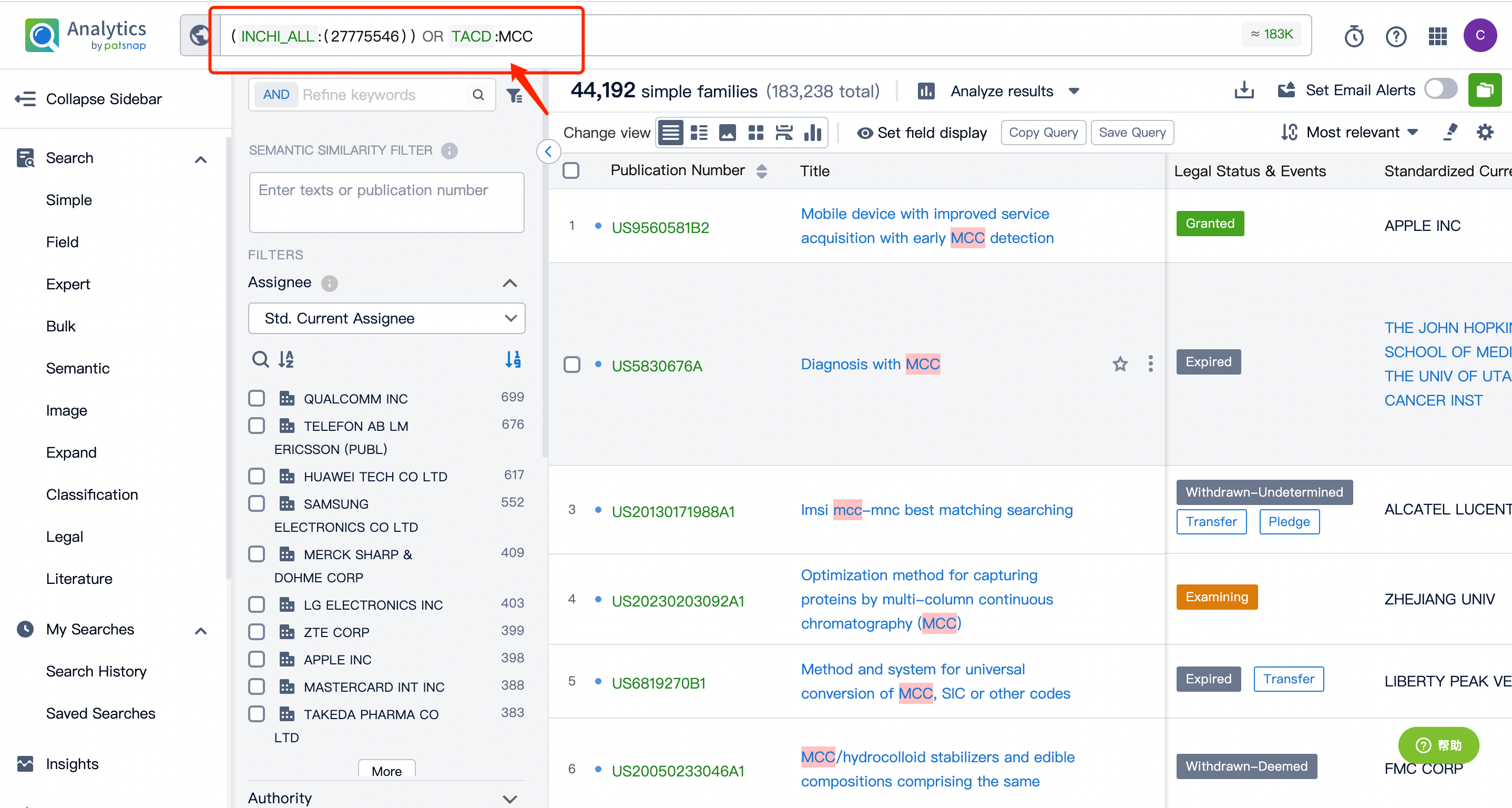
Afterward, go to the Chemical database to search for the patent query for Kadcyla's linker MCC and its cytotoxic agent DM1, and save them separately in the expert search library.
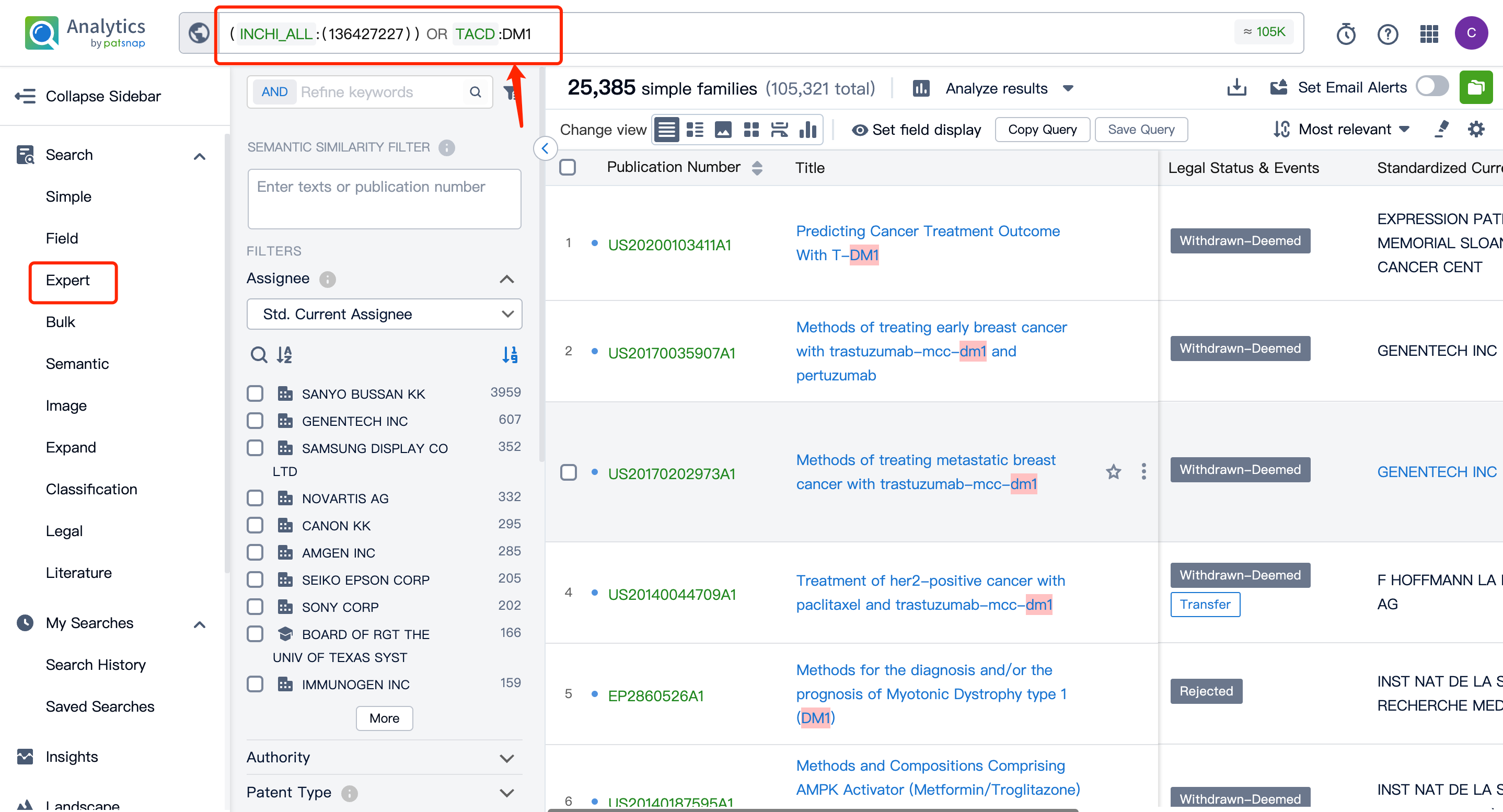
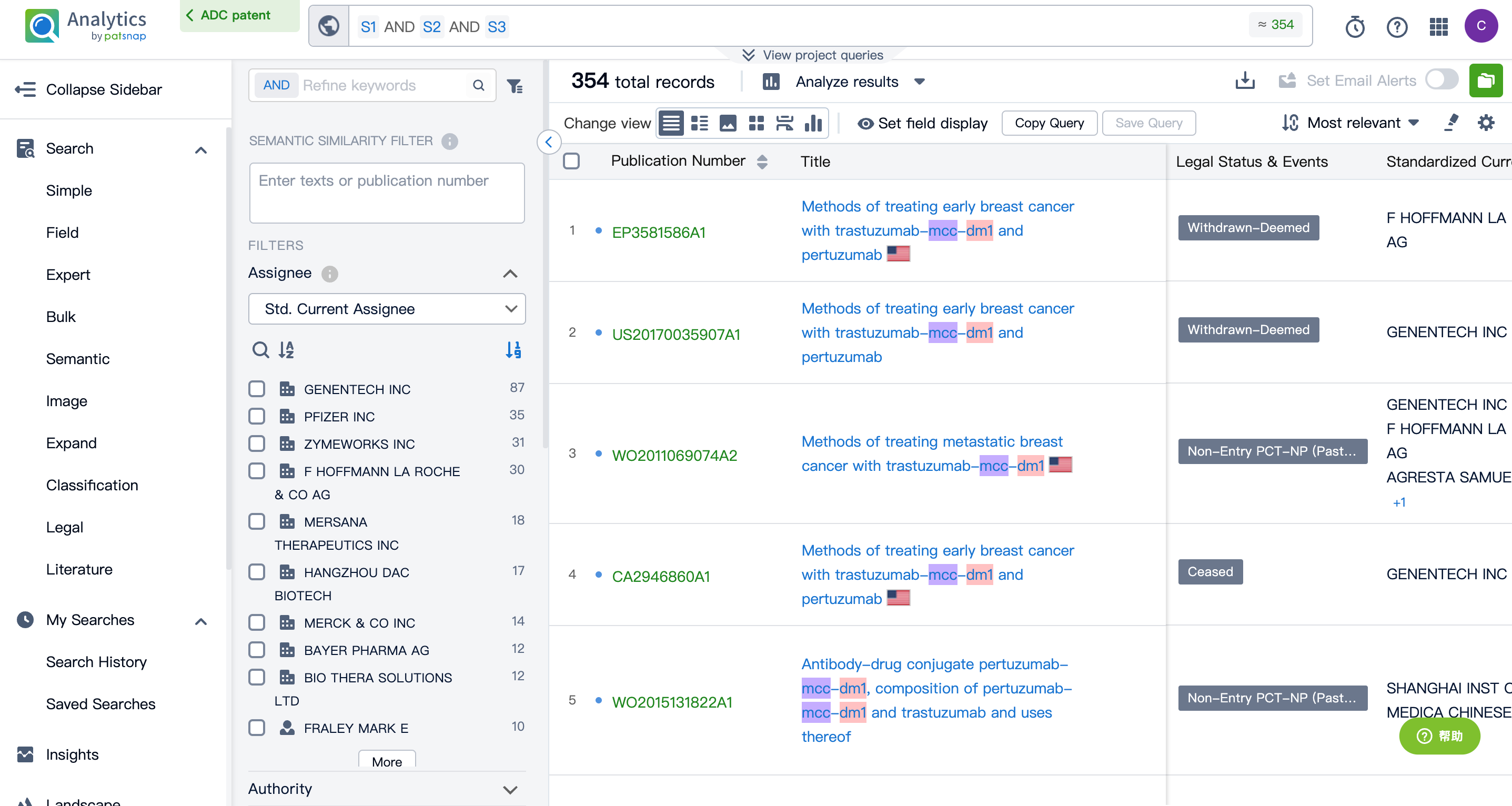
By performing an AND operation within the expert search library and combining the three retrieval formulas, one can generate the patent retrieval results for the ADC drug Kadcyla.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.