Unleashing the Power of Streptozocin: A Comprehensive Review on R&D Breakthroughs
Streptozocin's R&D Progress
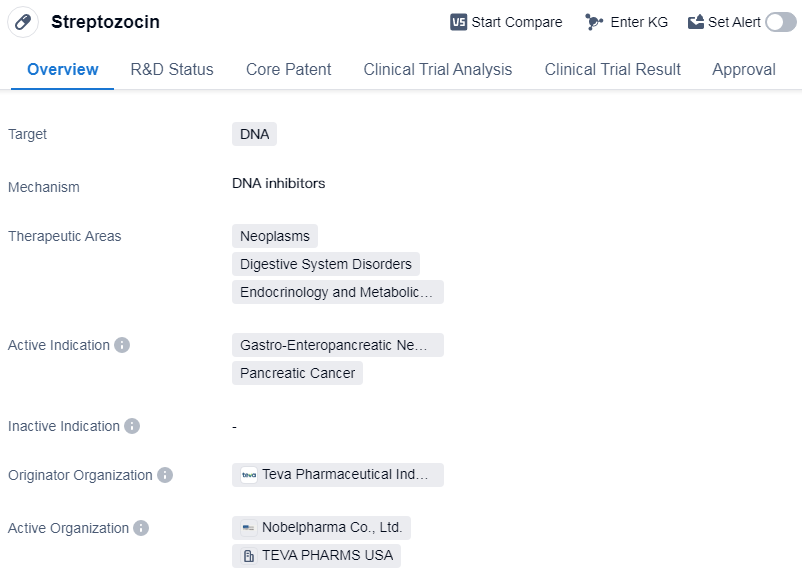
Streptozocin is a small molecule drug that primarily targets DNA. It is used in the treatment of various therapeutic areas including neoplasms, digestive system disorders, and endocrinology and metabolic diseases. The drug is specifically indicated for the treatment of gastro-enteropancreatic neuroendocrine tumor and pancreatic cancer.
Streptozocin was first approved for use in the United States in May 1982. It is classified as an orphan drug, which means it is intended to treat rare diseases or conditions that affect a small number of patients.
As a small molecule drug, Streptozocin is designed to interact with DNA, which plays a crucial role in the growth and replication of cells. By targeting DNA, the drug aims to disrupt the processes that contribute to the development and progression of neoplasms, digestive system disorders, and endocrinology and metabolic diseases.
Streptozocin has reached the highest phase of development, which is approval. This indicates that the drug has successfully completed clinical trials and has been deemed safe and effective for use in patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Streptozocin: DNA inhibitors
DNA inhibitors are substances or drugs that interfere with the replication or transcription process of DNA. These inhibitors can target various stages of DNA synthesis and function by binding to DNA or its associated proteins, thereby preventing the normal functioning of DNA. In the context of biomedicine, DNA inhibitors are often used as therapeutic agents to treat various diseases, particularly cancer.
In cancer treatment, DNA inhibitors are commonly used as chemotherapeutic drugs to inhibit the growth and division of cancer cells. By targeting the DNA replication process, these inhibitors can prevent cancer cells from proliferating and spreading. Examples of DNA inhibitors used in cancer treatment include topoisomerase inhibitors, which interfere with the action of enzymes involved in DNA replication, and antimetabolites, which disrupt the synthesis of DNA building blocks.
DNA inhibitors can also be used in research settings to study DNA replication, gene expression, and other cellular processes. By selectively inhibiting DNA replication or transcription, scientists can investigate the roles of specific genes or pathways in various biological processes.
It is important to note that while DNA inhibitors can be effective in treating certain diseases, they can also have side effects on normal cells that undergo rapid division. Therefore, the use of DNA inhibitors must be carefully monitored and managed by healthcare professionals.
Drug Target R&D Trends for Streptozocin
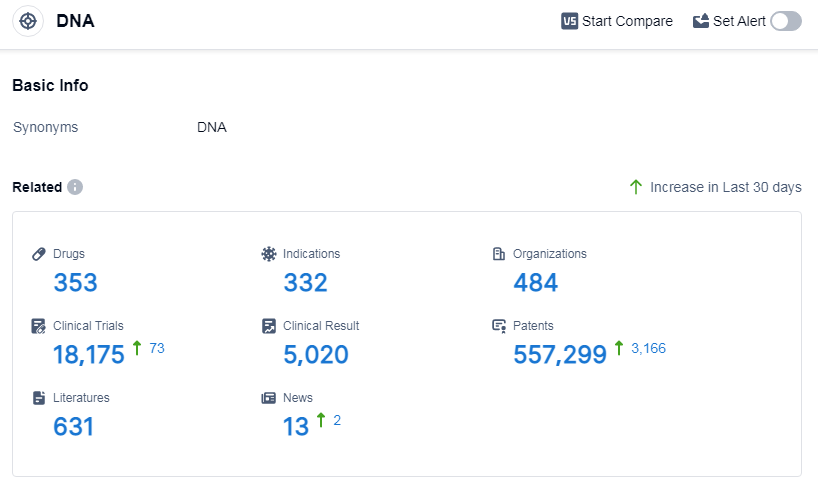
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 353 DNA drugs worldwide, from 484 organizations, covering 332 indications, and conducting 18175 clinical trials.
The analysis of the target DNA in the pharmaceutical industry reveals that Pfizer Inc., Novartis AG, Baxter International, Inc., Takeda Pharmaceutical Co., Ltd., and Johnson & Johnson are the companies growing fastest under this target. Lymphoma, ovarian cancer, and breast cancer are the indications with the highest number of approved drugs. Small molecule drugs, monoclonal antibodies, and antibody drug conjugates are the drug types progressing most rapidly. China, the United States, Japan, and the European Union are the countries/locations developing fastest under the target DNA. The future development of the target DNA holds promising opportunities for further advancements in drug development and treatment options.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Streptozocin is a small molecule drug that targets DNA and is used in the treatment of neoplasms, digestive system disorders, and endocrinology and metabolic diseases. Its primary indications are gastro-enteropancreatic neuroendocrine tumor and pancreatic cancer. The drug was first approved in the United States in 1982 and is classified as an orphan drug.