Rani Therapeutics begins Phase 1 trial of RT-111
Rani Therapeutics Holdings, Inc., a biotherapeutics enterprise at the clinical phase, keen on the oral administration of biologics and medications, proclaimed the launch of a Phase 1 clinical investigation assessing the safety and tolerability of RT-111. This orally provided RaniPill® GO capsule incorporates a CT-P43, which is an ustekinumab biosimilar. The preliminary findings of this study are scheduled to be available at the beginning of 2024's first quarter.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
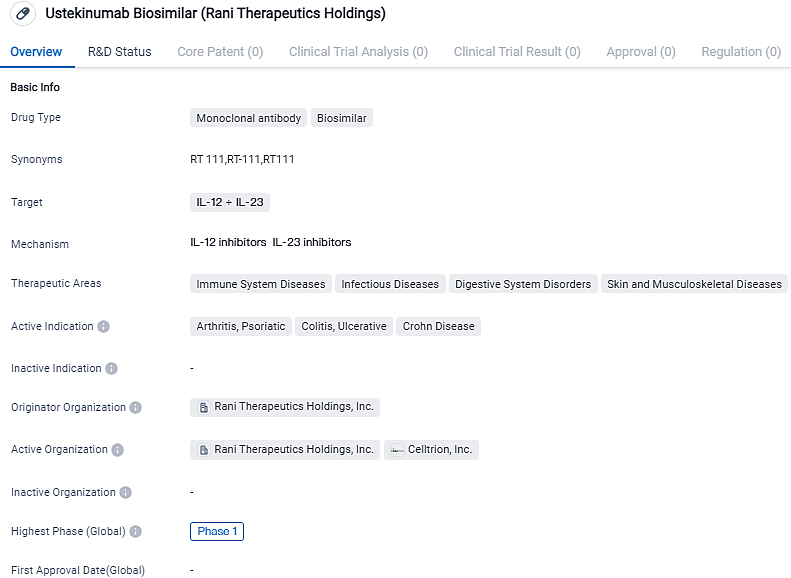
Ustekinumab, a human IgG1қ monoclonal antibody, specifically binds to the p40 protein subunit, which is utilised by both the interleukin-12 and interleukin-23 (IL-12 and IL-23) cytokines. In the United States, it's sold by Janssen under the brand name STELARA®.
The FDA has approved STELARA® for the management of moderate to severe plaque psoriasis, active psoriatic arthritis, moderate to severe Crohn's disease, and moderate to severe ulcerative colitis,. All of these conditions have a significant void in effective oral treatments. In 2022, STELARA® had an approximate revenue of $6.4 billion in the United States and roughly $9.7 billion globally.
Presently, ustekinumab can only be administered via a subcutaneous injection. However, during preclinical trials of RT-111 in animal models, the RaniPill® was successful in orally delivering a biosimilar of ustekinumab with bioavailability that rivals the subcutaneous injection.
“We're excited to share the progress of RT-111 into clinical trials, which signifies a critical breakthrough for Rani as we inch closer towards our vision of making oral biologics accessible for autoimmune disease patients,” stated Talat Imran, Rani's CEO. “RT-111, delivered orally via the RaniPill® capsule, offers a convenient method of administering ustekinumab. Furthermore, RT-111 also signals a potential wider scope to possibly supplant other injectable-only monoclonal antibodies and large molecules with an oral substitute.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
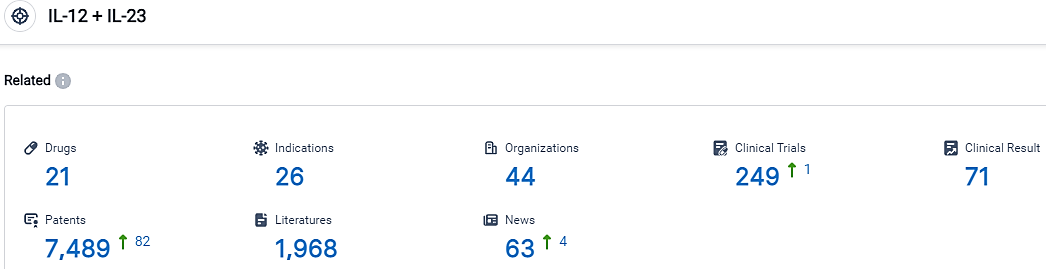
According to the data provided by the Synapse Database, As of September 25, 2023, there are 21 investigational drugs for the IL-12 and IL-23 target, including 26 indications,44 R&D institutions involved, with related clinical trials reaching 249,and as many as 7489 patents.
Ustekinumab Biosimilar targets IL-12 and IL-23 and is intended for the treatment of various immune system diseases, infectious diseases, digestive system disorders, as well as skin and musculoskeletal diseases. The active indications for this drug include arthritis, psoriatic, colitis, ulcerative, and Crohn's disease. Currently, Ustekinumab Biosimilar is in Phase 1 of clinical trials, indicating its progress towards potential approval.