KSQ Therapeutics Initiates Clinical Trials for CRISPR/Cas9 eTIL® Therapy KSQ-001EX with First Patient Dosed
KSQ Therapeutics, Inc. (KSQ), a biotechnology company in the clinical stage of developing therapies for solid tumors, has announced that the first patient has been treated in the Phase 1/2 trial of KSQ-001EX, an innovative edited TIL therapy. The KSQ-001EX therapy involves TIL where the SOCS1 gene has been deactivated using CRISPR/Cas9 gene editing technology, which may offer superior treatment potential for various solid tumor types.
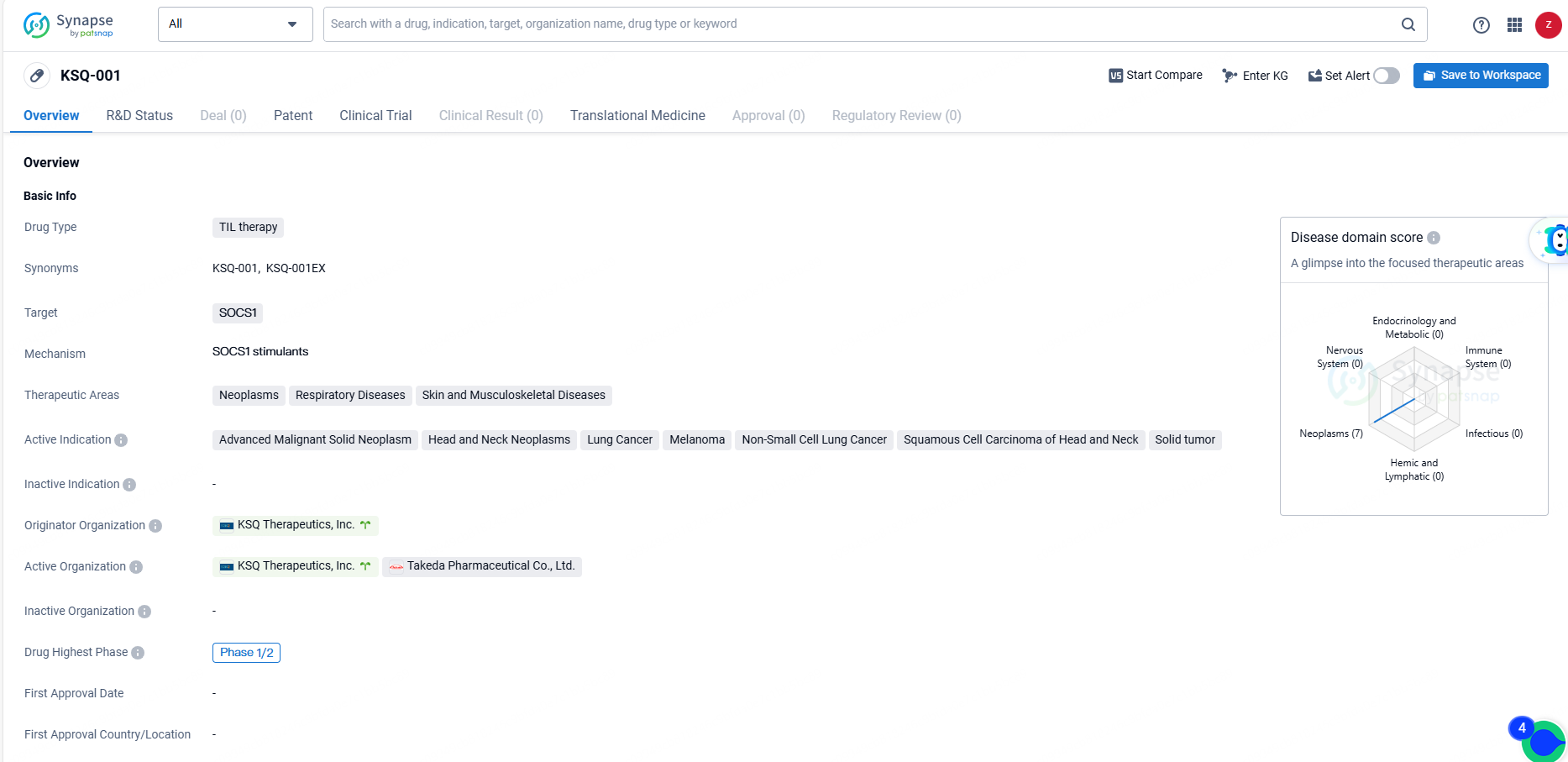
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
KSQ’s CRISPRomics platform identified SOCS1 as an essential gene that restricts T cell proliferation, survival, and differentiation by downregulating cytokine signaling within the tumor microenvironment.
"TILs have proven to be a highly effective new treatment approach, and we anticipate that both of our eTIL programs, KSQ-001EX and KSQ-004EX, could greatly contribute to the advancement of TIL therapy for various solid tumors. KSQ-001EX exhibited superior anti-tumor efficacy, polyclonality, persistence, and memory formation in preclinical models of multiple solid tumors compared to unaltered TILs. We also observed significant anti-tumor activity in models that were resistant to PD-1 blockade," stated Qasim Rizvi, CEO of KSQ.
Dr. Rodabe Amaria, a professor of Melanoma Medical Oncology and the principal investigator of the study at The University of Texas MD Anderson Cancer Center, oversaw the enrollment of the first patient treated with KSQ-001EX.
The Phase 1/2 clinical trial is an open-label study intended to assess safety at the outset for individuals with melanoma, head and neck squamous cell carcinoma (HNSCC), and non-small cell lung cancer. The main goal of the Phase 1 segment is to determine the safety and tolerability of KSQ-001EX, with an initial cohort of patients receiving the therapy without IL-2. In Phase 2, the primary aim is to assess anti-tumor activity within specific patient cohorts.
KSQ's foremost eTIL cell therapy program, KSQ-001EX, involves the modification of TILs to inactivate the SOCS1 gene and holds promise for transforming solid tumor treatments. Preclinical studies showed that KSQ-001EX improved anti-tumor performance in solid tumor models unresponsive to PD-1 inhibitors and also demonstrated increased persistence and memory formation.
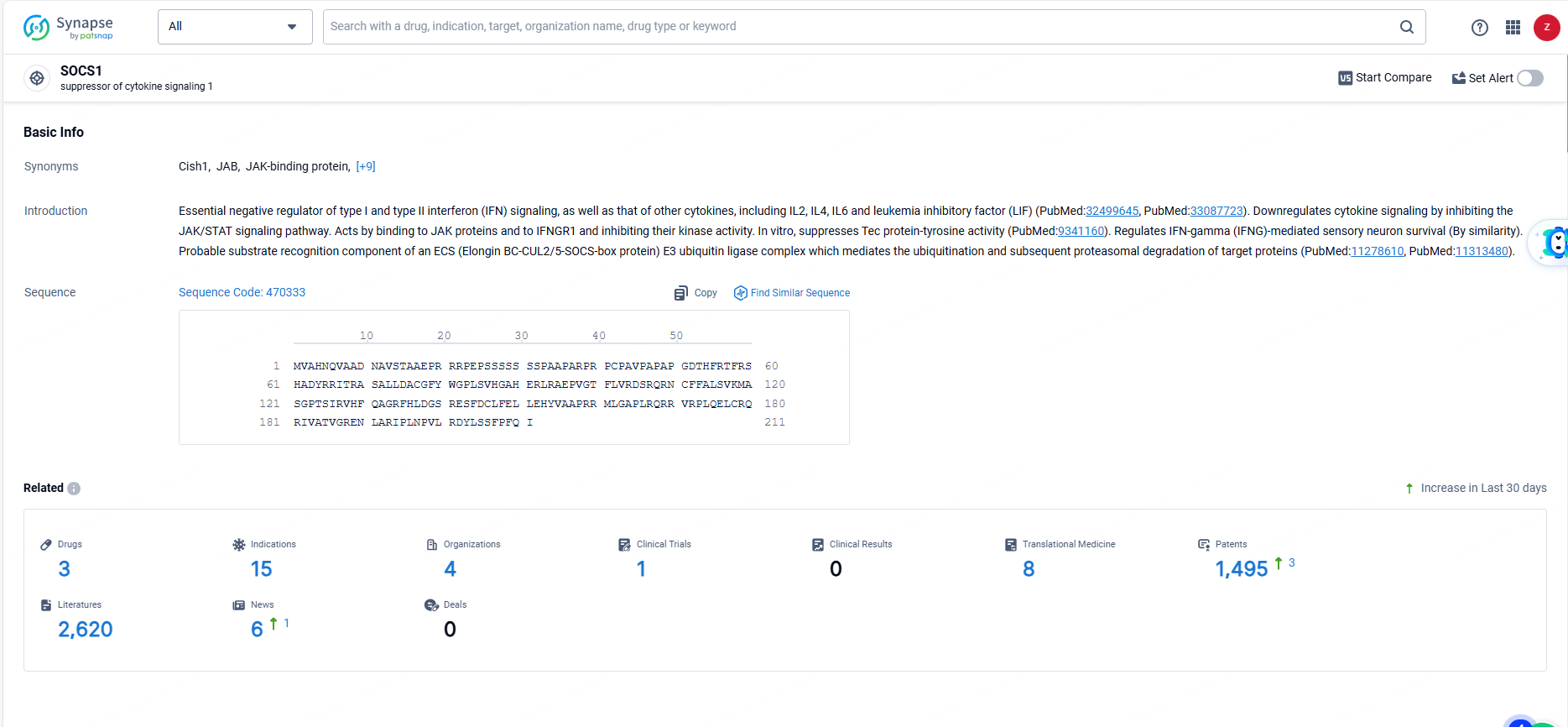
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 14, 2024, there are 3 investigational drugs for the SOCS1 targets, including 15 indications, 4 R&D institutions involved, with related clinical trials reaching 1, and as many as 1495 patents.
KSQ-001 is a TIL therapy drug targeting SOCS1, with active indications in various types of neoplasms and respiratory, skin, and musculoskeletal diseases. It is currently in Phase 1/2 clinical trials, and its development represents a promising advancement in the field of biomedicine.