Zumutor Biologics Reports First Patient Dosed with ZM008, an Innovative Anti-LLT1 Antibody
Zumutor Biologics Inc., a clinical-stage oncology firm situated in Boston which focuses on creating pioneering monoclonal antibody molecules targeting innate immune pathways, has recently disclosed that the initial patient has been treated in its Phase 1 clinical trial with ZM008. This dose-escalation study is investigating ZM008 in individuals with advanced solid tumors, both as a standalone treatment and alongside pembrolizumab.
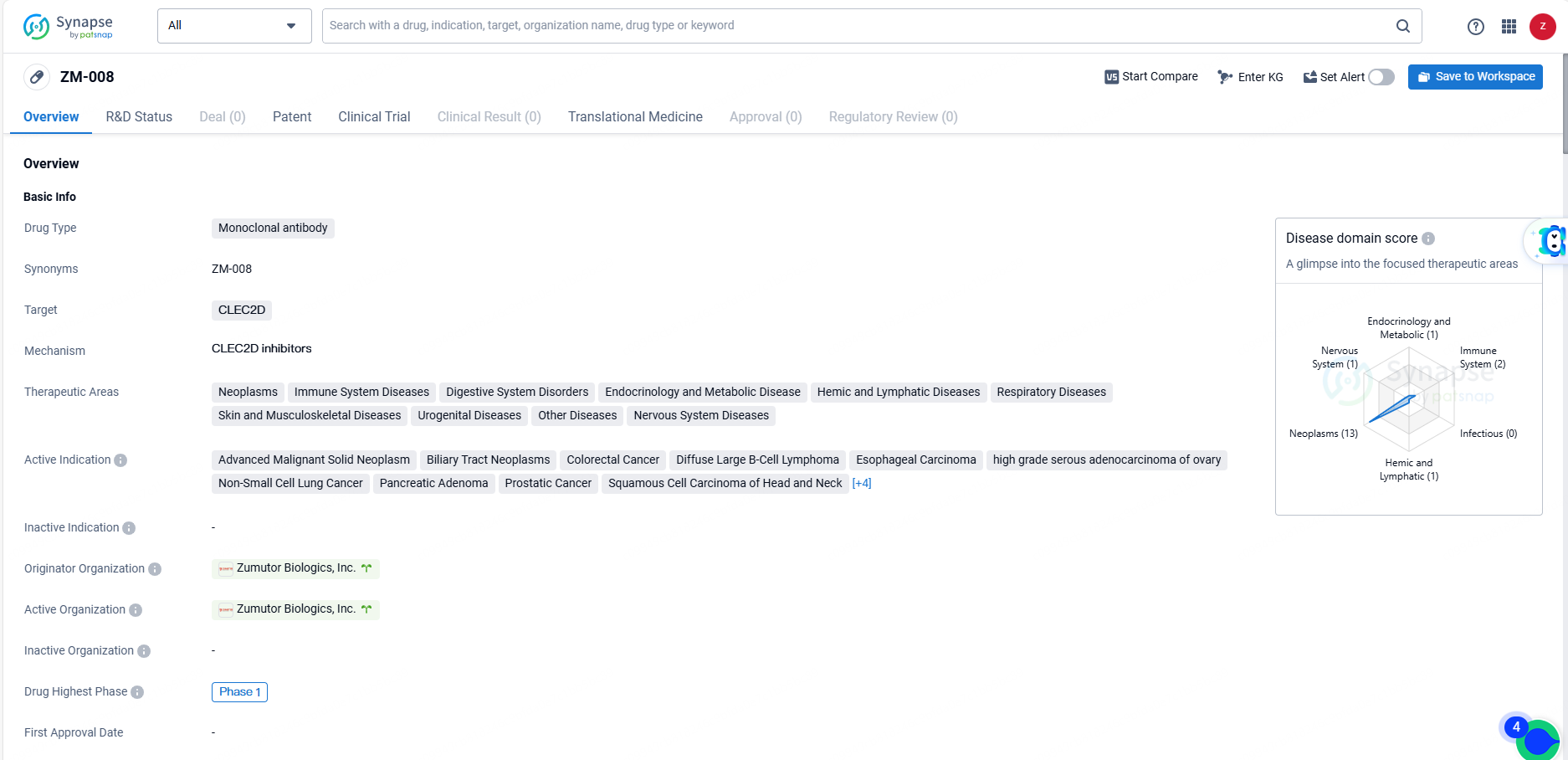
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
ZM008 is a groundbreaking fully human IgG1 monoclonal antibody targeting LLT1 (CLEC2D). It interferes with the LLT1-CD161 interaction between NK cells and tumor cells. This disruption activates NK cells, which subsequently triggers T cell activation, thereby altering the immune infiltrate within the tumor microenvironment to ultimately produce antitumor effects. The mechanism of action for ZM008 involves transforming ‘cold’ or less immune-responsive tumors into ‘hot’ or highly immune-responsive ones. This innovative approach could offer substantial benefits to patients who are resistant to existing immunotherapy options.
"Even though immunotherapy has significantly improved patient outcomes, there is still a substantial unmet need. ZM008 is a pioneering antibody that holds potential for aiding patients as a monotherapy as well as in conjunction with anti-PD-1 therapies. We are excited to be part of this study and hopeful that early clinical activity will be observed in this heavily pre-treated patient population, based on clinical and translational data," stated Dr. Ildefonso Ismael Rodriguez, MD, Principal Investigator at NEXT Oncology.
The ZM008-001 trial is an open-label, first-in-human, multicenter, Phase 1 dose escalation study of ZM008 administered either alone or alongside Pembrolizumab. The trial aims to evaluate the safety, pharmacokinetics, maximum tolerated dose, pharmacodynamic biomarkers, and preliminary antitumor activity of ZM008. The study will determine the recommended dose for Phase 2.
In the initial stage (1A), escalating doses of ZM008 will be administered to patients with solid tumors who have no standard therapeutic options. This will be followed by stage 1B, where ZM008 will be given in combination with the anti-PD-1 drug, Pembrolizumab.
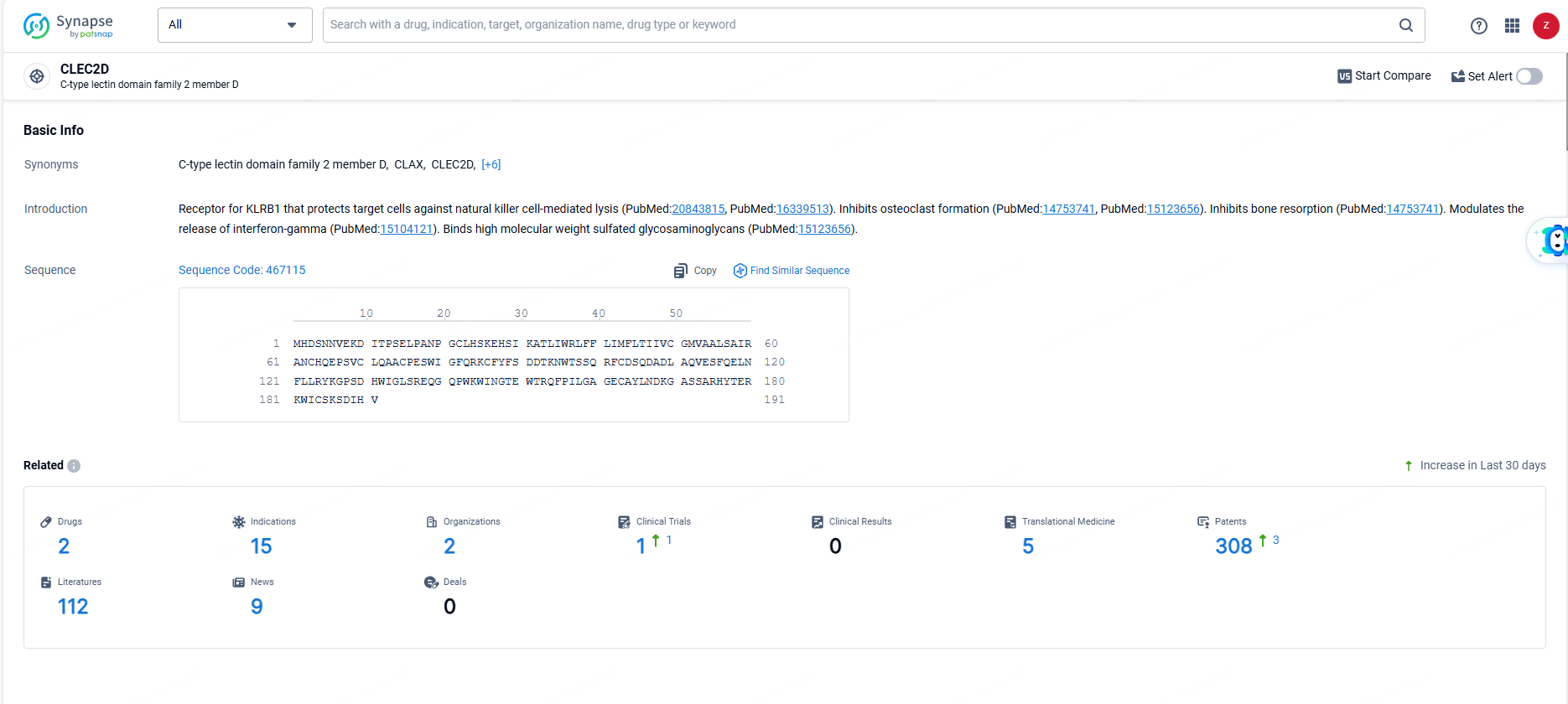
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 14, 2024, there are 2 investigational drugs for the CLEC2D targets, including 15 indications, 2 R&D institutions involved, with related clinical trials reaching 1, and as many as 308 patents.
ZM-008 represents a promising monoclonal antibody drug with a broad potential for treating various diseases across different therapeutic areas. Its specific targeting of CLEC2D and its diverse range of active indications make it a candidate for addressing unmet medical needs in multiple disease areas. As the drug progresses through clinical development, it will be important to closely monitor its efficacy and safety profile in order to determine its potential for future commercialization and patient benefit.