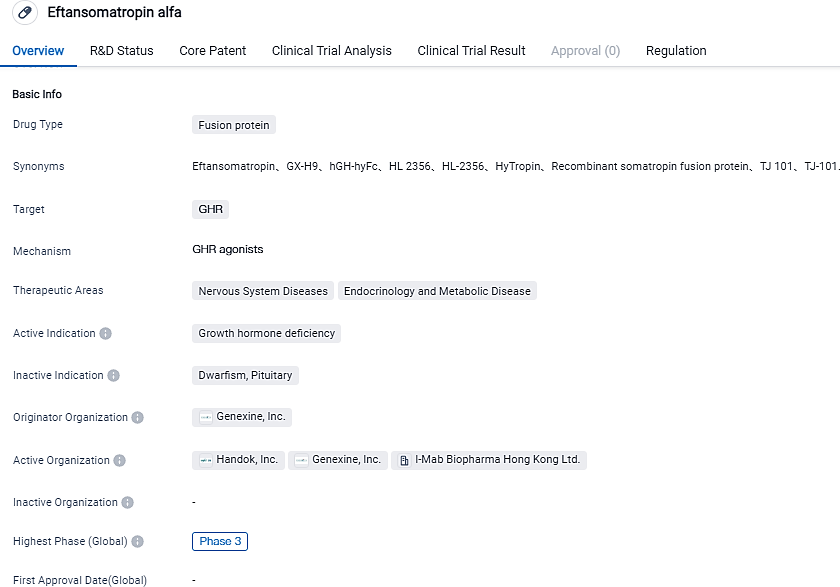
Genexine's long-lasting growth hormone successfully reaches primary endpoint of Phase 3 trial
Genexine, a listed clinical-stage biopharmaceutical corporation based in Korea that is dedicated to discovering and developing innovative biologics for addressing unmet medical demands, shared optimistic primary results today from the diverse-center, randomized, open-label, active-controlled key phase 3 research (CTJ101PGHD301) happening in China, assessing the efficacy and security of eftansomatropin alfa (an exclusive long-acting growth hormone by Genexine, also referred to as GX-H9) in children suffering from growth hormone deficiency.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
I-Mab, the collaborator of Genexine in China, has successfully completed a study which showed that eftansomatropin alfa effectively met the set criterion of yearly height growth rate at the end of a year and proved it to be as efficient as Norditropin®. Eftansomatropin alfa was applied through a weekly injection, contrary to the everyday dosage of Norditropin®. The typical AHV came to 10.76 (cm/year) with eftansomatropin alfa as opposed to 10.28 (cm/year) with Norditropin®, indicating a discrepancy of 0.47 [95% CI -0.06,1.00]. The p-value of non-inferiority was less than 0.0001. Eftansomatropin alfa showed good tolerability with no drug discontinuation reports due to associated adverse effects. Its safety shared similarity with that of Norditropin®. The organization intends to make a BLA submission in China in 2024.
Genexine's CEO and President, Neil Warma, expressed his satisfaction after the results of this registration trial were out, which revealed safety and efficiency of this potential superior medication for treating growth hormone deficiency in children. Warma said, "Consider this as a significant step for Genexine as it shows our capacity to introduce our innovative products to the market as well as the worth of our exclusive long-acting hyFc®platform. We look forward to filing the BLA submission in 2024 and bringing this product to the Chinese market and patients. We persist in our endeavors to bring our leading commodities to several important markets."
About long-acting recombinant human growth hormone or Eftansomatropin alfa
GX-H9 was formulated using Genexine's hyFc®platform, aiming to treat growth hormone deficiency and it is at present being assessed in a phase 3 registration trial in China for treating pediatric GHD, with an expected BLA submission in 2024. GX-H9 is differentiated by its prolonged half-life which necessitates significantly fewer injections besides its superior safety profile. Pediatric patient compliance has notably improved. GX-H9 is co-developed through a strategic partnership with Handok and I-Mab for development in the wider China region. I-Mab has partnered with Jumpcan, looking ahead to launch and commercialize eftansomatropin alfa in China.
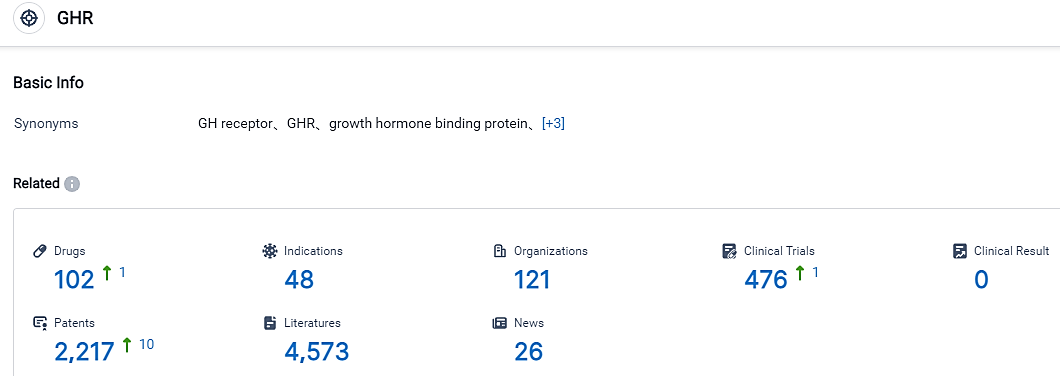
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the information disclosed by the Synapse Database, as of September 1, 2023, there are 102 investigational drugs for the GHR target, including 48 applicable indications, 121 R&D institutions involved, with related clinical trials reaching 476, and as many as 2217 patents.
Genexine, Inc. is a publicly listed biotechnology firm in the clinical stage, concentrating on the development and market introduction of immunotherapeutics and cutting-edge long-acting biologics. Its primary technology platforms are Therapeutic DNA vaccine technology and hyFc®fusion technology. They have a multitude of products in the clinical development phase, some of which are on the verge of Phase 3 registration trials. The company's exclusive pipeline includes products like GX-188E (tirvalimogene teraplasmid) for treating head, neck and cervical cancer, GX-I7 (efineptakin alfa) for various types of cancer, GX-H9 (eftansomatropin alfa) intended for Pediatric Growth Hormone Deficiency and GX-E4 for CKD-induced anemia, among others.
Genexine has instituted multiple global partnerships to facilitate and speed up the development and market introduction of these products. With its headquarters in Seoul, Korea, it is listed on the Korean exchange. Genexine fervently serves the wellbeing and care of patients everywhere in the world.