Germany's Merck KGaA enhances its oncology portfolio by partnering strategically with Hengrui for advanced PARP1 inhibitor and antibody-drug conjugate
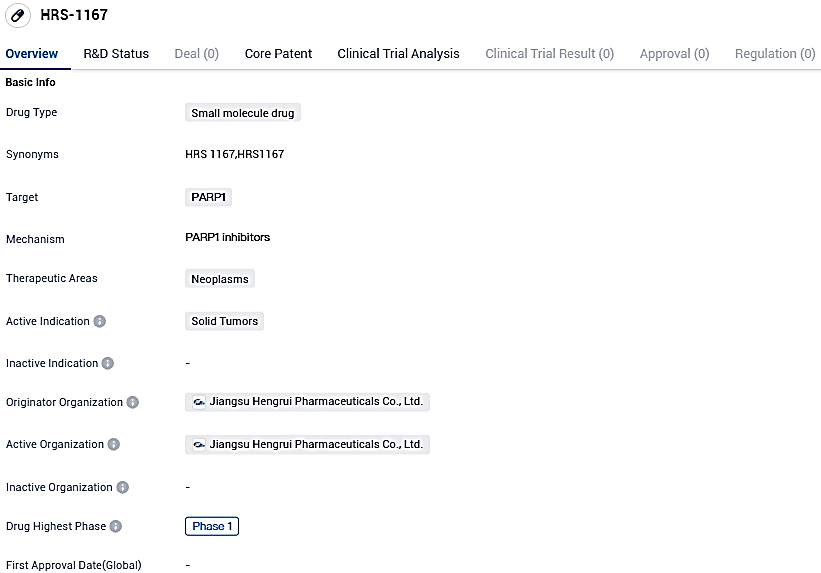
Germany's top science and technology firm, Merck KGaA, Darmstadt, has declared a strategic alliance with Jiangsu Hengrui Pharmaceuticals Co. Ltd.. In this collaboration, a global exclusive license is given to devise, produce, and market Hengrui’s advanced, potent, and selective PARP1 trapping inhibitor HRS-1167.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The contract also provisions an exclusive worldwide license opportunity to develop, manufacture, and market Hengrui’s Claudin-18.2 antibody-drug conjugate SHR-A1904. Additionally, Merck KGaA, based in Darmstadt, Germany, holds the right to collectively promote both products in China.
"Cohorting with Hengrui is in perfect harmony with our external innovation vision and cancer research and development approach, fortifying our strong internal pipeline in the fields of DNA damage response inhibition and antibody-drug conjugates," stated Danny Bar-Zohar, Global Director of Research & Development and Chief Medical Counsel for Merck KGaA's Healthcare division, located in Darmstadt, Germany.
Compared to the initial generation of PARP inhibitors, the specificity and distinct safety attributes of PARP1-specific inhibitors may provide an avenue to extend their medicinal implications in established and novel applications.
HRS-1167 has demonstrated promising indications of clinical efficacy and patient advantages in Phase I studies when used alone and shows an enhanced propensity for integration with chemotherapy and innovative agents versus earlier attempts using initial generation PARP inhibitors.
This aligns with the corporate’s holistic approach of DNA damage response inhibitors, investigating their collective efficacy and maximizing their potential effect in combination with other anti-cancer therapies and strategies. Merck KGaA, Darmstadt, Germany, is conducting research into the potential of treating cancer by inhibiting several targets in the DDR cascade, including ATR, ATM, DNA-PK, and PARP.
In accordance with the agreement’s stipulations, Merck KGaA, Darmstadt, Germany, will offer Hengrui a one-time upfront compensation of €160 million. Hengrui will receive additional payments following the accomplishment of certain product development, regulatory approvals, and commercial milestones, along with a tiered royalty system on net sales conducted by Merck KGaA, Darmstadt, Germany. Potential payments could reach a maximum total of €1.4 billion.
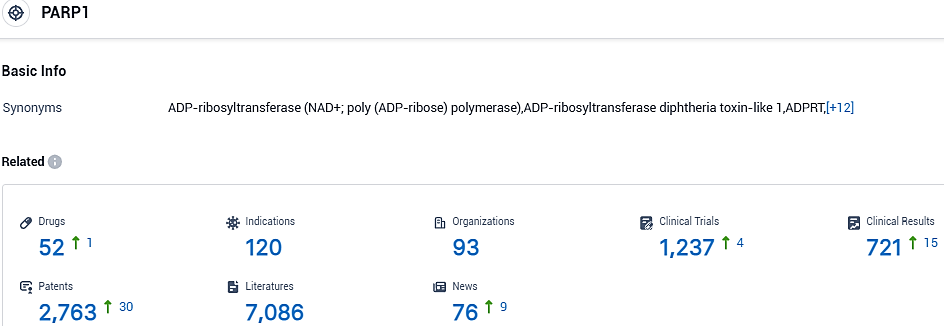
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 1, 2023, there are 52 investigational drugs for the PARP1 target, including 120 indications, 93 R&D institutions involved, with related clinical trials reaching 721, and as many as 2763 patents.
HRS-1167 displays potential for interfering with DNA repair processes within cancerous cells. Additional investigation and refinement are required to ascertain its effectiveness and safety in a broader patient group.