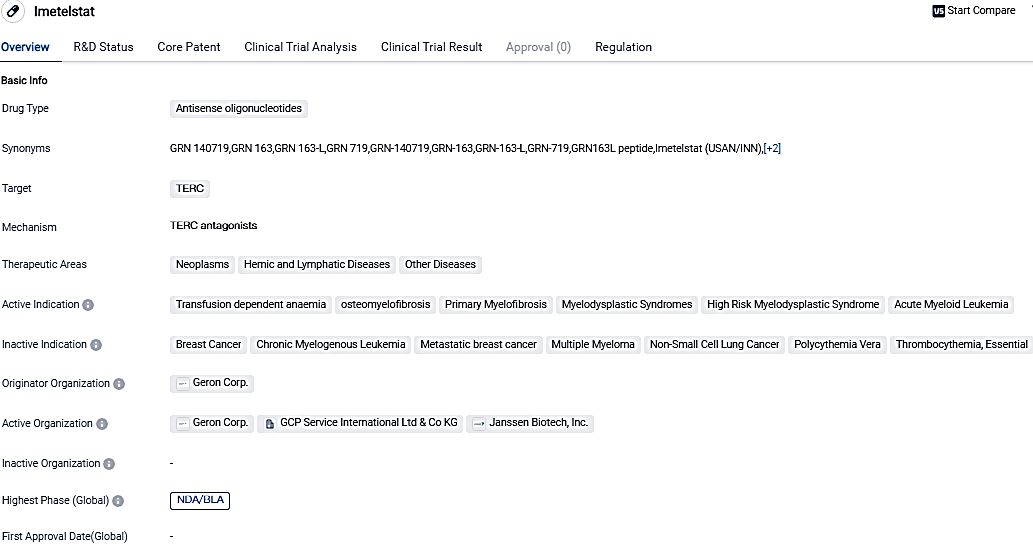
Geron Reports Validation by EMA of Marketing Approval Submission for Imetelstat
The biopharmaceutical company, Geron Corporation, which performs advanced clinical research, declared that the Marketing Authorization Application concerning imetelstat-a novel experimental telomerase inhibitor-has been accepted and validated by the EMA. This application seeks to utilize imetelstat in managing transfusion-dependent anemia, specifically amongst patients diagnosed with lower-risk myelodysplastic syndromes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Earlier this month, the MAA was presented and is presently undergoing evaluation by the European Committee for Medicines for Human Use, in accordance with the centralized procedure. This process is applicable to all 27 EU nations as well as Iceland, Norway, and Liechtenstein. The MAA assessment is predicted to conclude in roughly 14 months.
John A. Scarlett, M.D., Geron's CEO and Chairman stated that the EMA's validation of the marketing authorization application for imetelstat brings them a step closer to potentially providing this first-of-kind treatment to low-risk MDS patients experiencing anemia in the EU. He added that given imetelstat's clinical record so far, they are hopeful for it to become a routine treatment and solve longstanding unfulfilled requirements of lower risk MDS patients.
The basis for the MAA are results from IMerge Phase 3, where the primary goal of 8-week transfusion independence was notably higher with imetelstat in comparison to placebo, with the median TI duration nearing a year for 8-week TI respondents treated with imetelstat. Average hemoglobin levels in patients treated with imetelstat considerably improved over time in comparison to those on placebo.
Key MDS subgroups experienced statistically notable and clinically valuable efficacy results, regardless of ring sideroblast status, IPSS risk category or baseline transfusion burden. Patient-reported outcome data indicated a maintained significant improvement in fatigue for imetelstat-treated patients in contrast to placebo. Consistent with prior imetelstat clinical experiences, the majority of serious adverse events were mostly short-lived, manageable cytopenias.
Imetelstat, a novel and unique telomerase inhibitor owned solely by Geron, is under development for hematologic malignancies. Non-clinical studies and clinical trials involving imetelstat have provided substantial proof that the agent targets telomerase to restrict the rampant growth of malignant stem and progenitor cells in myeloid hematologic cancers, resulting in malignant cell apoptosis and potential disease-altering activity.
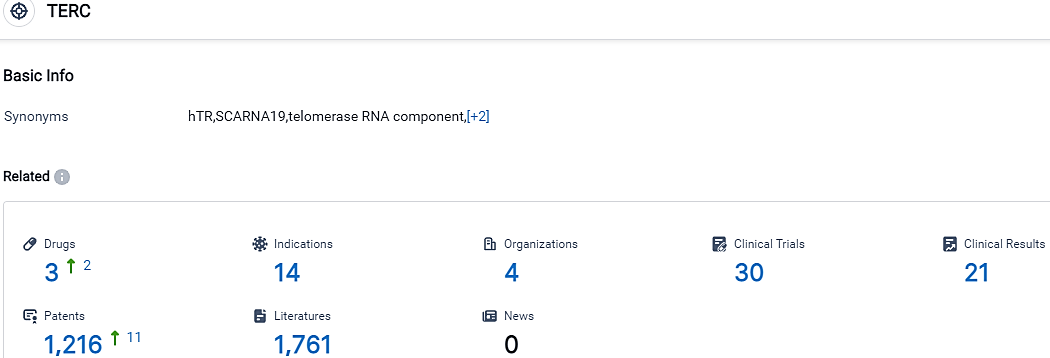
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 9, 2023, there are 3 investigational drugs for the TERC target, including 14 indications, 4 R&D institutions involved, with related clinical trials reaching 30,and as many as 1216 patents.
Imetelstat has been granted Fast Track designation by the U.S. Food and Drug Administration for both the treatment of adult patients with transfusion dependent anemia. The MAA validation follows the acceptance by the U.S. FDA of a New Drug Application, which was assigned a Prescription Drug User Fee Act action date of June 16, 2024.