New Hope for Solid Tumor Treatment: TIL Therapy
Immunotherapy, especially cell-based immunotherapy, has brought new strategies for cancer treatment in the past decade. Recent clinical research has shown that the cell transfer therapy of tumor infiltrating lymphocytes (TILs) exhibits good efficacy in patients with late-stage solid tumors. TIL therapy uses the patient's own immune cells from the solid tumor microenvironment to kill tumor cells.
There have been many methods for immunotherapy to treat cancer, such as immune checkpoint inhibitors, CAR-T cell therapy, and so forth. However, CAR-T cell therapy has not yet been proven to be effective on the treatment of solid tumors. Therefore, further research on TIL therapy is of significant importance for the treatment of patients of various types of solid tumors.
Compared with other cell immunotherapies, TIL therapy has unique advantages. TILs consist of T cells from multiple TCR clones, which can directly target shared self-antigens, react to tumor-specific neoantigens, and thus more effectively deal with the heterogeneity of tumors. In addition, TILs usually contain a large amount of effector memory T cells, which, after being stimulated by tumor antigens, will express chemokine receptor, making it easier to locate in tumor tissues after infusion.
TIL therapy presents some limitations. Firstly, to achieve a sustained anti-tumor response, there needs to be a presence of effector T cells with anti-tumor activity, however, many solid tumors lack such cells. Furthermore, the immunosuppressive tumor microenvironment could lead to the exhaustion of infiltrating cytotoxic T cells, reducing their ability to clear cancer cells. The selection and expansion of TILs also pose challenges and require further improvement.
In order to overcome the limitations of TIL therapy, new treatment strategies need to be explored. A potential option is to employ NK cells or tumor-infiltrating γδT cells to combat solid tumors. In addition, interventions against the immunosuppressive microenvironment are also crucial, such as increasing the density and maturity of TA-HEV endothelial cells to promote T cell infiltration, or using combination therapies to enhance the efficacy of TIL therapy.
TIL therapy, as a cell-based immunotherapy approach, displays some unique advantages in the treatment of solid tumors. However, the limitations and challenges still need to be addressed. By exploring new treatment strategies, improving TIL selection and expansion techniques, and intervening in the immunosuppressive tumor microenvironment, we hope to further enhance the efficacy of TIL therapy, offering new hope for patients with solid tumors.
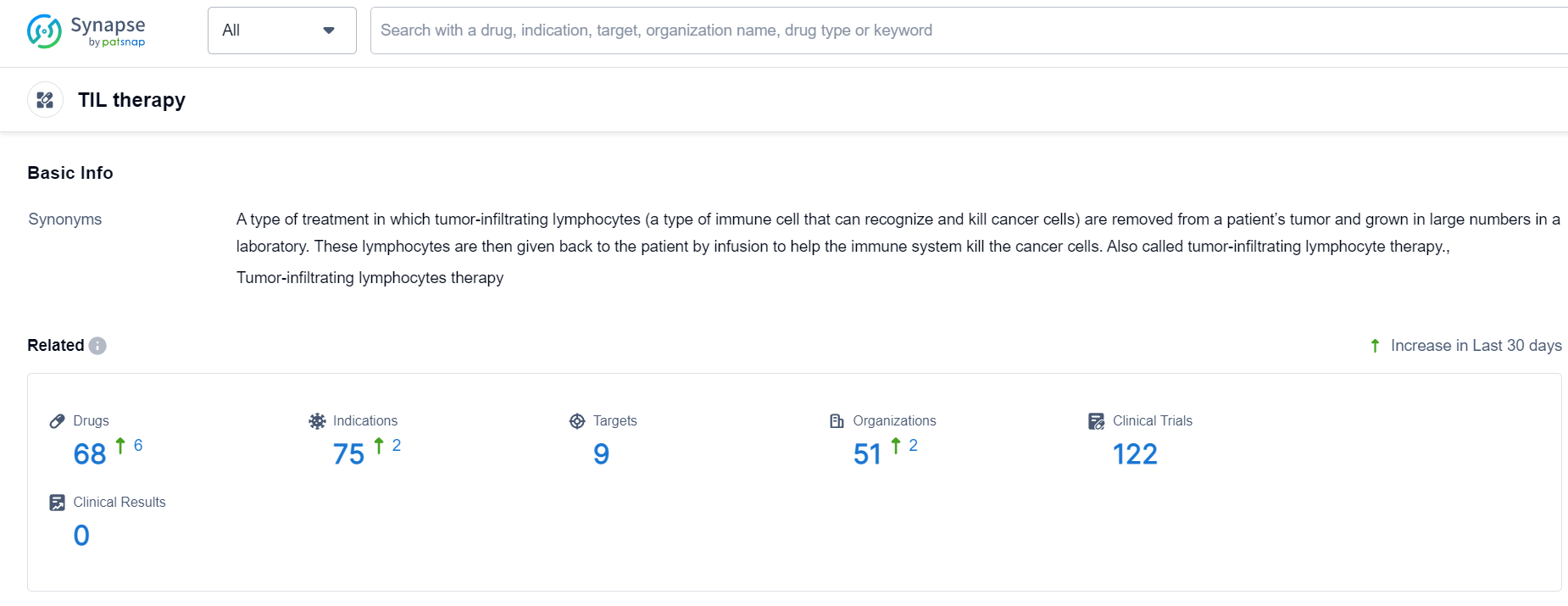
TIL therapy Competitive Landscape
According to Patsnap Synapse, as of 24 Sep 2023, there are a total of 68 TIL therapy drugs worldwide, from 51 organizations, covering 9 targets, 75 indications, and conducting 122 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
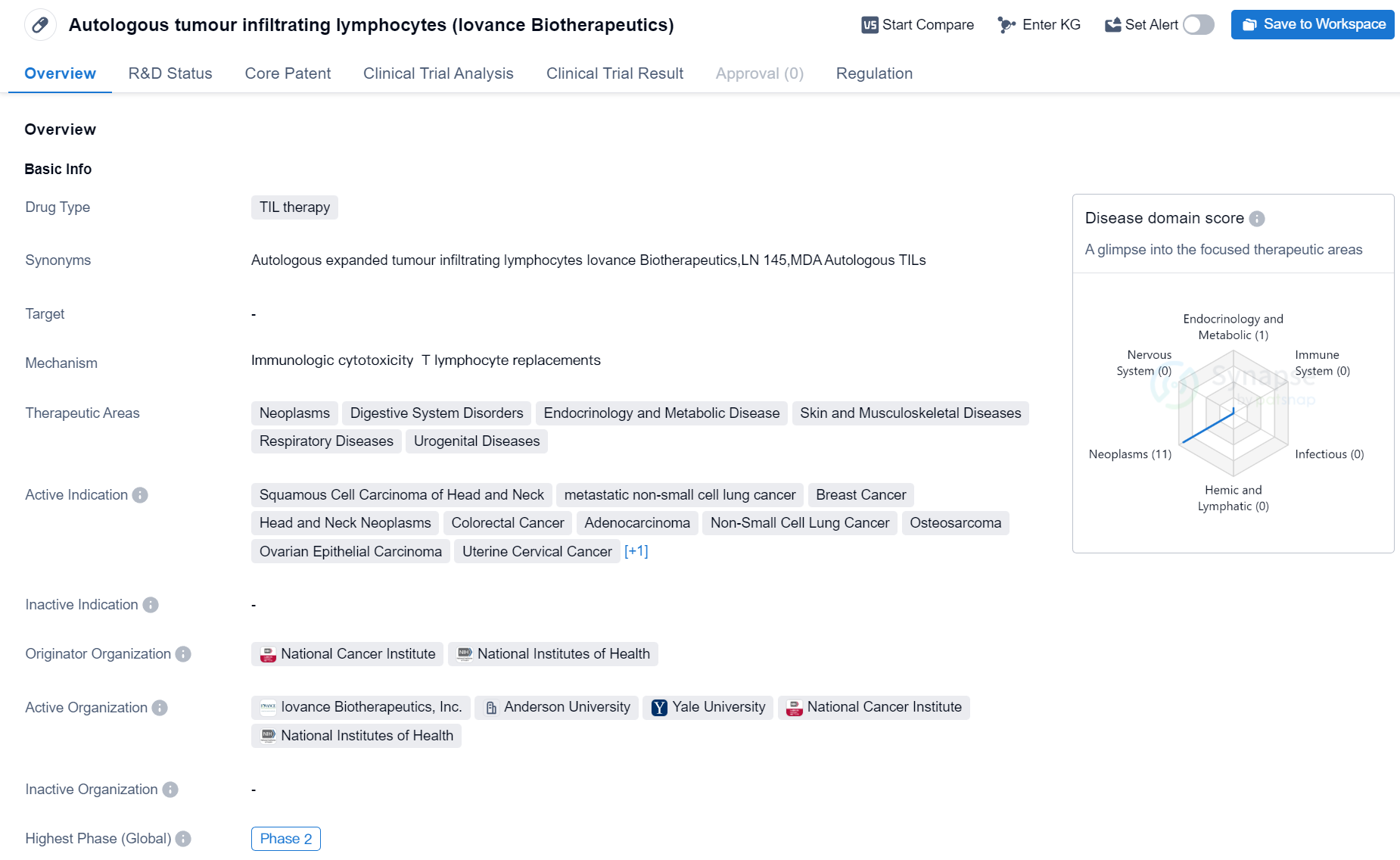
Phase II Clinical Trial of TIL therapy Medicinal: Autologous tumour infiltrating lymphocytes
Autologous tumour infiltrating lymphocytes (TIL) therapy is a drug currently in development in the field of biomedicine. It is being studied for its potential use in treating various types of cancers, including Squamous Cell Carcinoma of Head and Neck, metastatic non-small cell lung cancer, Breast Cancer, Head and Neck Neoplasms, Colorectal Cancer, Adenocarcinoma, Non-Small Cell Lung Cancer, Osteosarcoma, Ovarian Epithelial Carcinoma, Uterine Cervical Cancer, and Melanoma.
The drug is being developed by Iovance Biotherapeutics, and its originator organizations are the National Cancer Institute and the National Institutes of Health. Currently, the drug is in Phase 2 of clinical trials, which means it has already undergone initial testing for safety and efficacy and is now being further evaluated in a larger group of patients.
Autologous TIL therapy has been granted regulatory designations of Fast Track and Breakthrough Therapy. Fast Track designation is given to drugs that treat serious conditions and have the potential to address unmet medical needs, while Breakthrough Therapy designation is granted to drugs that show substantial improvement over existing treatments for life-threatening conditions.
The therapeutic areas targeted by this drug include Neoplasms (cancer), Digestive System Disorders, Endocrinology and Metabolic Disease, Skin and Musculoskeletal Diseases, Respiratory Diseases, and Urogenital Diseases. This suggests that the drug has the potential to be used in a wide range of medical conditions beyond just cancer.
In summary, Autologous TIL therapy is a drug currently in Phase 2 of clinical trials, being developed by Iovance Biotherapeutics in collaboration with the National Cancer Institute and the National Institutes of Health. It is being studied for its potential use in treating various types of cancers, and it has received regulatory designations of Fast Track and Breakthrough Therapy. The drug has the potential to address unmet medical needs in multiple therapeutic areas, making it an exciting prospect in the field of biomedicine.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
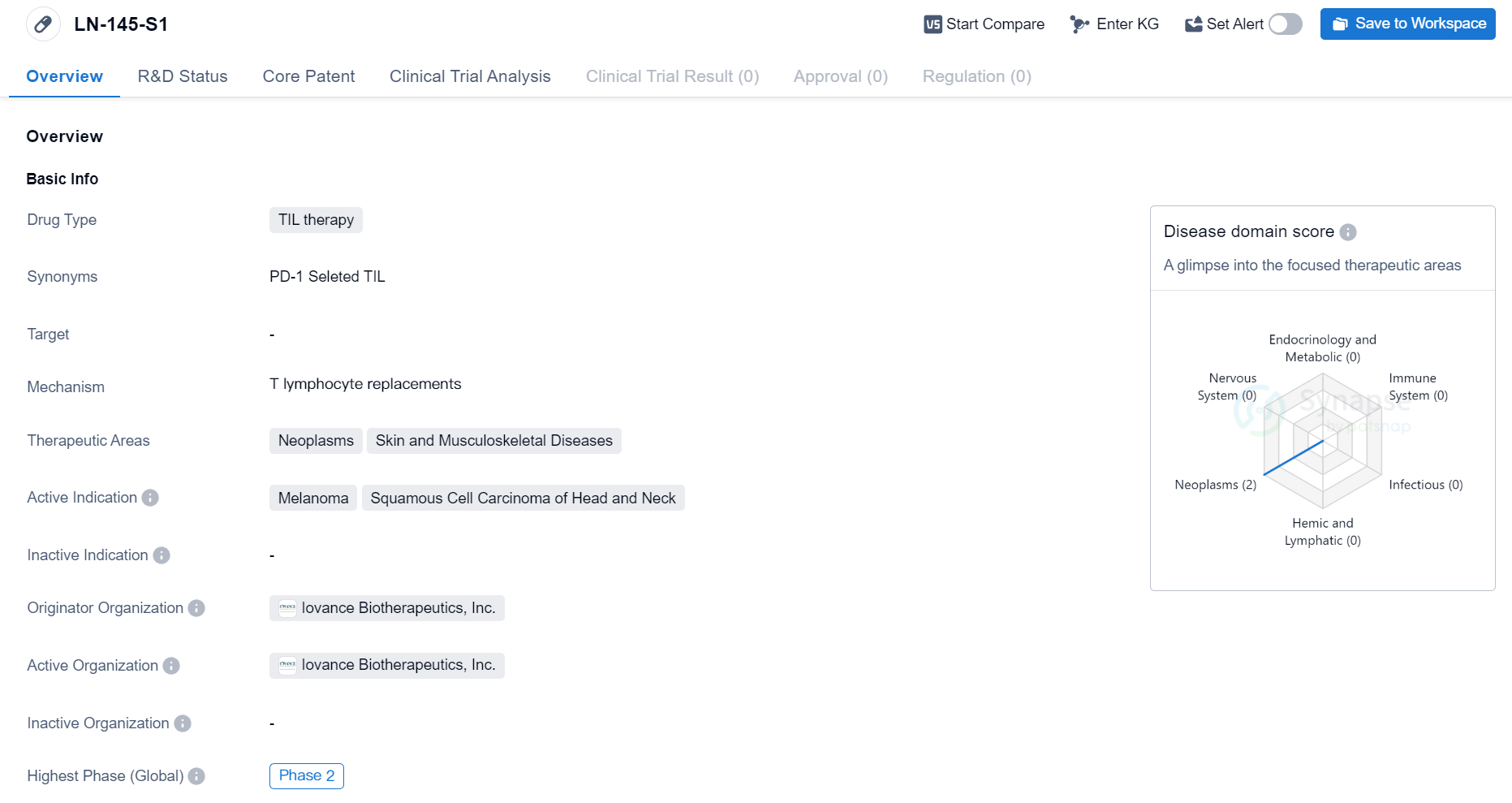
Phase II Clinical Trial of TIL therapy Medicinal: LN-145-S1
The drug LN-145-S1 is a type of TIL therapy, which stands for tumor-infiltrating lymphocyte therapy. TIL therapy is a form of immunotherapy that involves extracting immune cells from a patient's tumor, expanding them in the laboratory, and then reinfusing them back into the patient to target and destroy cancer cells.
The therapeutic areas for LN-145-S1 are neoplasms (abnormal growth of cells) and skin and musculoskeletal diseases. This suggests that the drug is primarily being developed to treat various types of cancer, particularly melanoma and squamous cell carcinoma of the head and neck. Melanoma is a type of skin cancer that develops from pigment-producing cells, while squamous cell carcinoma is a type of cancer that arises from the squamous cells, which are flat cells that line the surface of the skin and other organs.
The drug is being developed by Iovance Biotherapeutics, Inc., a pharmaceutical company specializing in the development of novel cancer immunotherapies. As the originator organization, Iovance Biotherapeutics is responsible for the research, development, and commercialization of LN-145-S1.
Based on the information provided, LN-145-S1 is currently in the phase 2 of clinical development. Clinical trials are conducted in multiple phases to evaluate the safety and efficacy of a drug in humans. Phase 2 trials typically involve a larger number of patients and aim to further assess the drug's effectiveness and side effects.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, LN-145-S1 is a TIL therapy being developed by Iovance Biotherapeutics for the treatment of neoplasms, specifically melanoma and squamous cell carcinoma of the head and neck. The drug is currently in Phase 2 of clinical development, indicating that it has shown promising results in earlier stages of testing and is being further evaluated for its safety and efficacy.