GigaGen Begins Phase 1 Trial, Administers First Dose of GIGA-564 to Solid Tumor Patient
GigaGen Inc., a biotechnology firm focused on developing groundbreaking antibody therapies for immune deficiencies, infectious diseases, and cancers resistant to checkpoint inhibitors, and a subsidiary of Grifols, has revealed that they have administered the initial dose to a patient in a Phase 1 clinical trial. This trial is designed to assess the safety and tolerability of their anti-CTLA-4 oncology drug, GIGA-564, for the treatment of metastatic or locally advanced solid tumors.
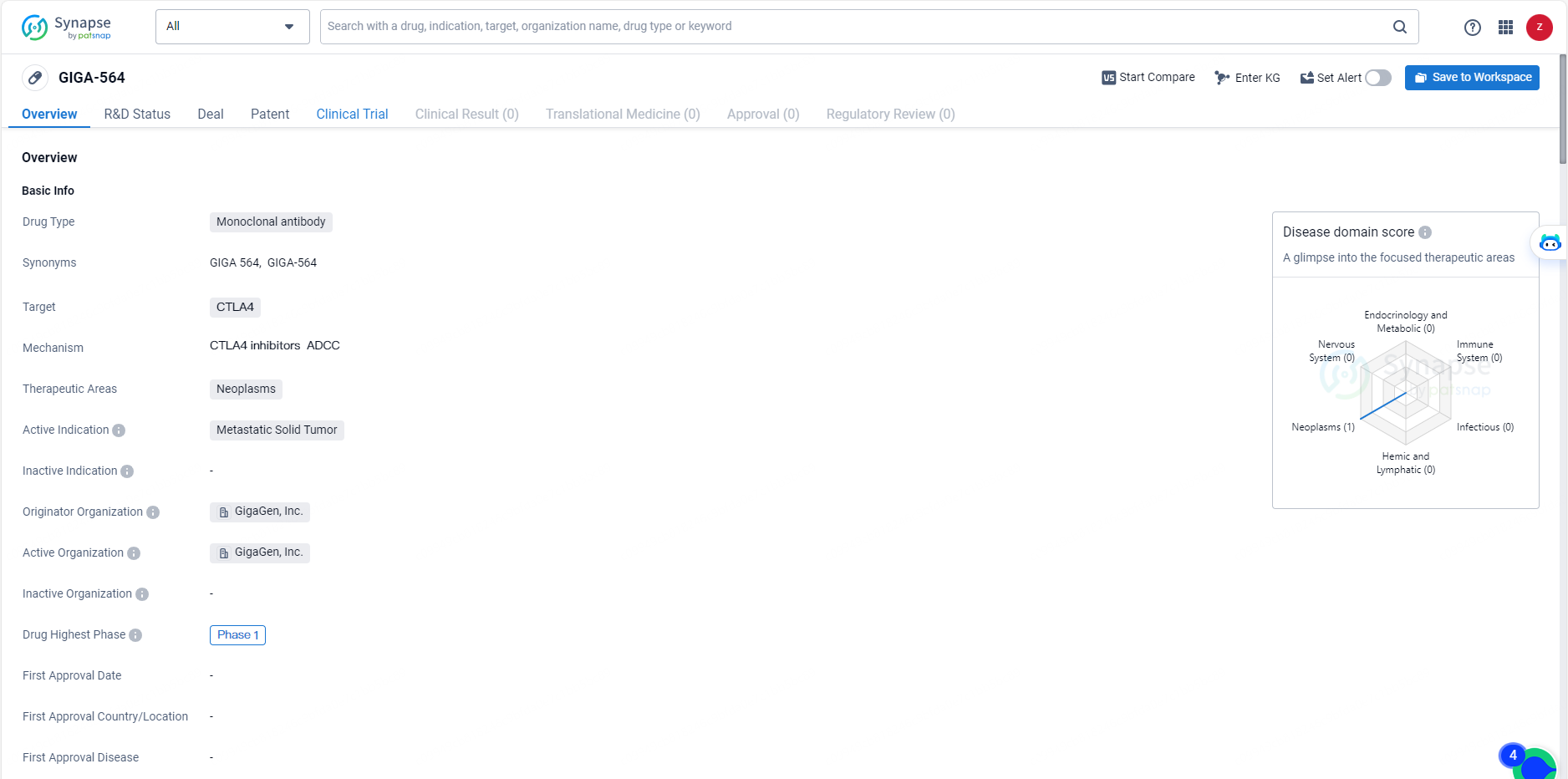
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"The commencement of this Phase 1 trial featuring GIGA-564 signifies an important milestone as it represents GigaGen’s initial oncology asset entering the clinical phase," stated Carter Keller, senior vice president at Grifols and leader of GigaGen. "We are eager to potentially transform the exceptional anti-tumor efficacy and diminished immune-related toxicity exhibited by GIGA-564 in pre-clinical evaluations into a clinical reality. There is a critical demand for novel therapies targeting solid tumors and we are optimistic that GIGA-564 could enhance patient outcomes."
This trial is being executed by researchers at the National Cancer Institute, a division of the National Institutes of Health, in close collaboration with GigaGen. For detailed information about the trial, please refer to clinicaltrials.gov identifier: NCT06258304.
GIGA-564, a fully human monoclonal antibody, sets itself apart from existing anti-CTLA-4 drugs. Previous anti-CTLA-4 medications were engineered to robustly inhibit CTLA-4’s interaction with its ligands, consequently amplifying T cell co-stimulation. Nevertheless, this method has been linked with increased immune-related adverse effects.
Additionally, recent discoveries indicate that previous anti-CTLA-4 drugs facilitate the expansion of T regulatory cells, which could reduce their intended effect of activating cytotoxic T cells essential for targeting tumors. In contrast, GIGA-564’s distinctiveness lies in its limited CTLA-4 inhibition and its efficacy in depleting intratumoral Tregs within the tumor environment.
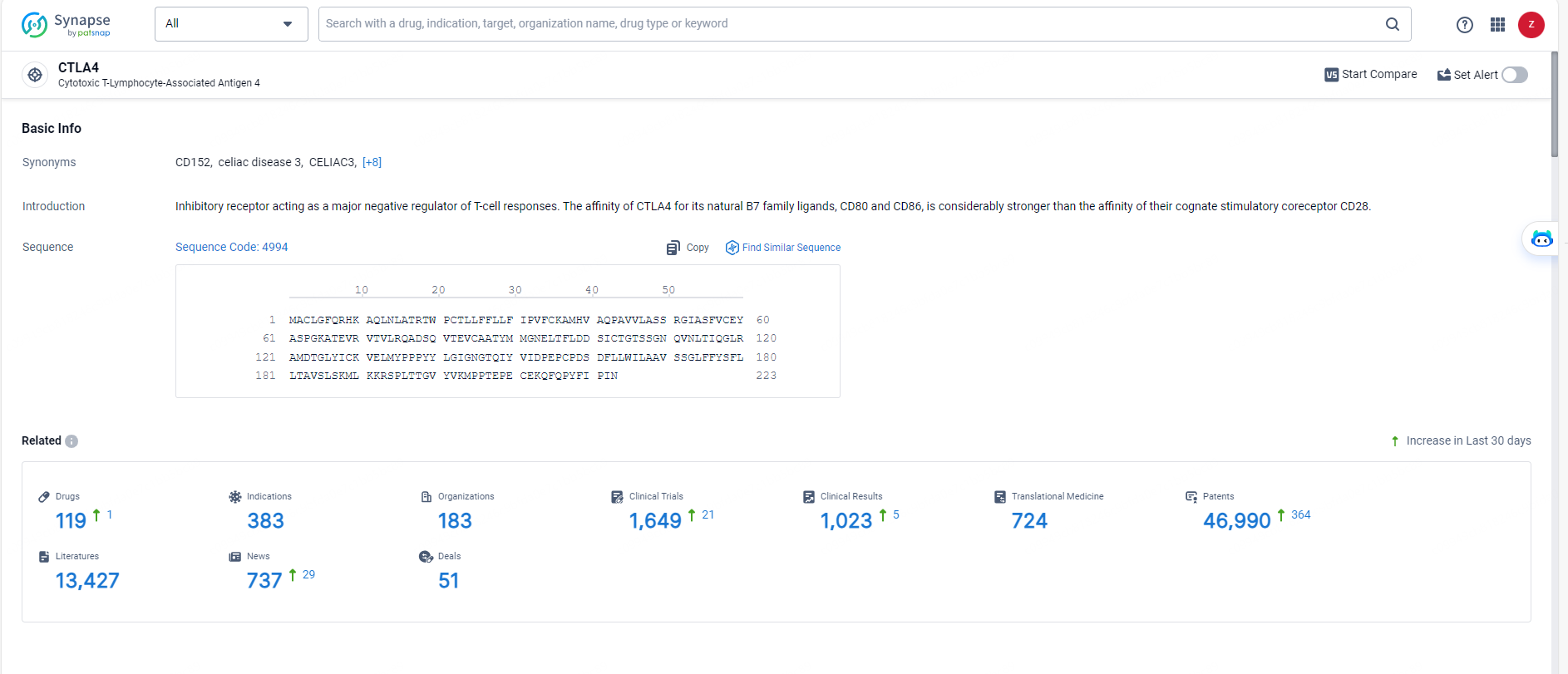
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 27, 2024, there are 119 investigational drugs for the CTLA4 targets, including 383 indications, 183 R&D institutions involved, with related clinical trials reaching 1649, and as many as 46990 patents.
GIGA-564 is a monoclonal antibody drug targeting CTLA4 for the treatment of neoplasms, specifically metastatic solid tumors. With its advancement to Phase 1 clinical trials and its potential to modulate the immune response in the context of cancer, GIGA-564 represents a significant development in the field of biomedicine and holds promise as a potential treatment option for patients in need.