Glycopyrrolate: Detailed Review of its Transformative R&D Success, Mechanism of Action
Glycopyrrolate's R&D Progress
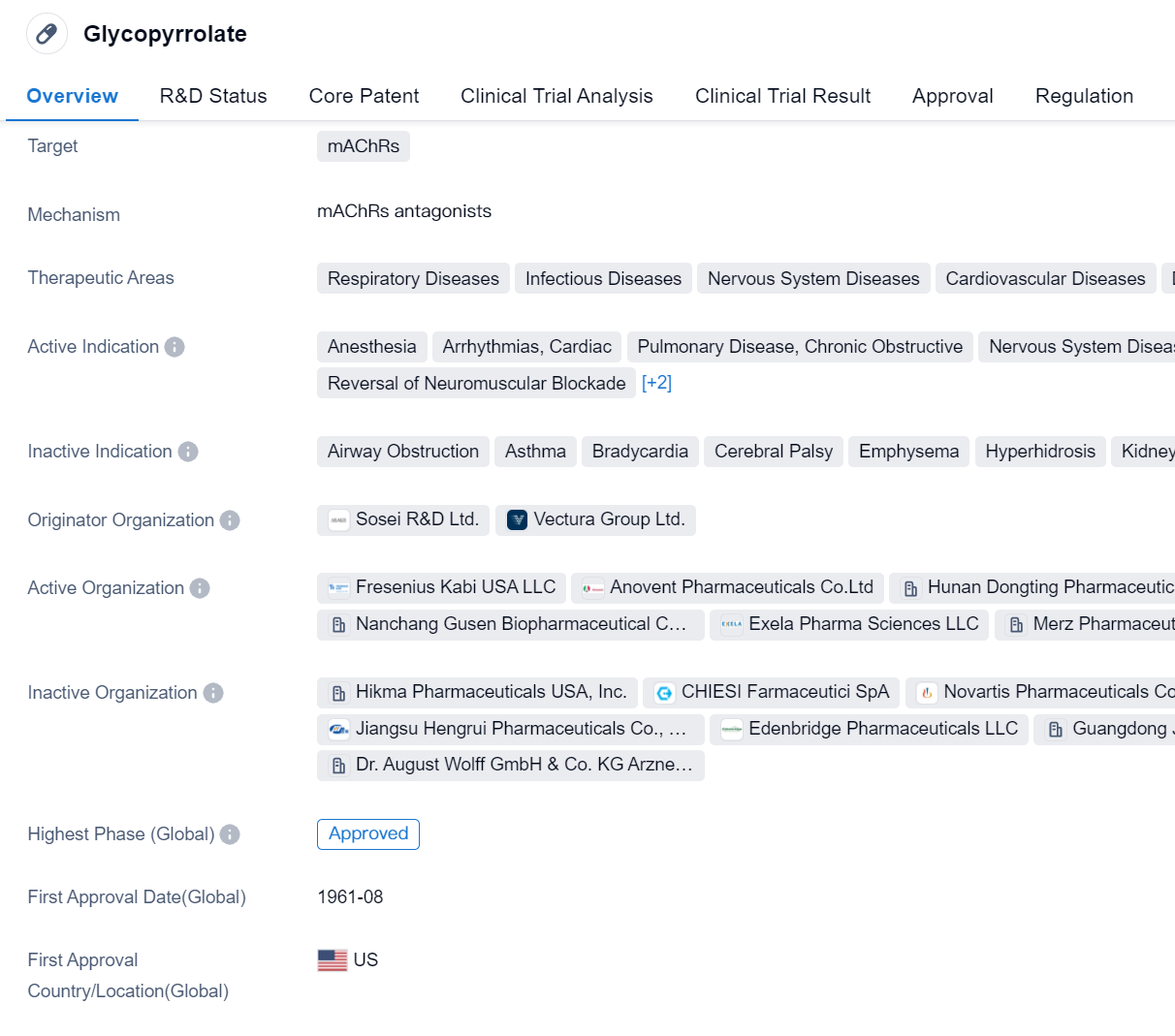
Glycopyrrolate is a small molecule drug that targets mAChRs (muscarinic acetylcholine receptors). It has been approved for use in various therapeutic areas, including respiratory diseases, infectious diseases, nervous system diseases, cardiovascular diseases, digestive system disorders, mouth and tooth diseases, and other diseases.
The active indications for Glycopyrrolate include anesthesia, arrhythmias (cardiac), chronic obstructive pulmonary disease (COPD), nervous system diseases, sialorrhea (excessive drooling), duodenal ulcer, gastritis, stomach ulcer, peptic ulcer, reversal of neuromuscular blockade, chronic bronchitis, and pulmonary emphysema.
Glycopyrrolate was first approved in the United States in August 1961, making it a well-established drug with a long history of use. It was developed by Sosei R&D Ltd. and Vectura Group Ltd., both of which are originator organizations in the pharmaceutical industry.
In terms of regulatory status, Glycopyrrolate has received priority review and orphan drug designation. Priority review is a regulatory pathway that expedites the review process for drugs that address unmet medical needs or provide significant improvements over existing treatments. Orphan drug designation is granted to drugs that are intended to treat rare diseases or conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Glycopyrrolate: mAChRs antagonists
mAChRs antagonists refers to muscarinic acetylcholine receptor antagonists. Muscarinic acetylcholine receptors (mAChRs) are a type of G protein-coupled receptors found in the central and peripheral nervous systems. They are activated by the neurotransmitter acetylcholine.
Antagonists, in the context of pharmacology, are substances that bind to a receptor and block its activation. Therefore, mAChRs antagonists are drugs or compounds that bind to muscarinic acetylcholine receptors and prevent the activation of these receptors by acetylcholine.
From a biomedical perspective, mAChRs antagonists are commonly used in the treatment of various conditions such as overactive bladder, chronic obstructive pulmonary disease (COPD), and Parkinson's disease. By blocking the activation of mAChRs, these antagonists can help alleviate symptoms associated with excessive cholinergic activity, such as urinary urgency, bronchoconstriction, and tremors.
It is important to note that mAChRs antagonists can have side effects due to their broad effects on the cholinergic system. These side effects may include dry mouth, blurred vision, constipation, and cognitive impairment. The specific mAChRs antagonist used and its dosage will depend on the targeted condition and the individual patient's needs.
Drug Target R&D Trends for Glycopyrrolate
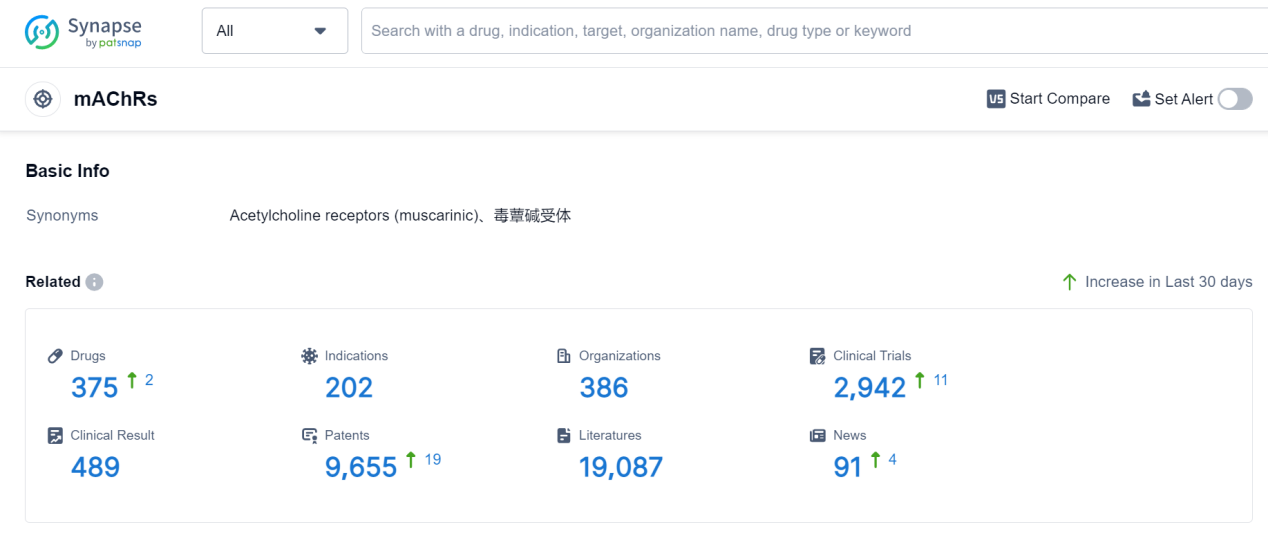
Muscarinic acetylcholine receptors (mAChRs) are a class of G-protein coupled receptors found in various tissues throughout the human body. They play a crucial role in mediating the effects of acetylcholine, a neurotransmitter involved in numerous physiological processes. mAChRs are involved in regulating heart rate, smooth muscle contraction, glandular secretion, and neuronal signaling in the central and peripheral nervous systems. Dysfunction of mAChRs has been implicated in various diseases, including Alzheimer's disease, Parkinson's disease, and asthma. Understanding the role of mAChRs is essential for developing targeted therapies that modulate their activity, potentially leading to improved treatments for these conditions.
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 375 mAChRs drugs worldwide, from 386 organizations, covering 202 indications, and conducting 2942 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Glycopyrrolate is a widely used drug that has been approved for multiple indications and has a long history of use. Its approval in both the global markets indicates its broad applicability and potential benefits for patients. The drug's targeting of mAChRs suggests its mechanism of action involves modulating the activity of these receptors, which may have therapeutic effects in various disease areas. The specific therapeutic areas and indications for Glycopyrrolate highlight its potential in treating a wide range of conditions, including respiratory, cardiovascular, and digestive system disorders.