GSK Reports Positive Phase III Results from ANCHOR Trials on Depemokimab for Chronic Sinusitis with Nasal Polyps
GSK plc (LSE/NYSE: GSK) has revealed encouraging primary findings from the phase III trials ANCHOR-1 and ANCHOR-2, which evaluated the safety and effectiveness of depemokimab compared to a placebo in adults suffering from CRSwNP. Both studies achieved their co-primary goals, showing a significant change from baseline in overall endoscopic nasal polyp scores after 52 weeks and alterations in the mean nasal obstruction scores from weeks 49 to 52. The frequency and severity of treatment-related adverse events were comparable in both the depemokimab group and the placebo group throughout both trials. Further examination of these results is currently in progress. Comprehensive findings from ANCHOR-1 and ANCHOR-2 will be shared at an upcoming scientific conference.
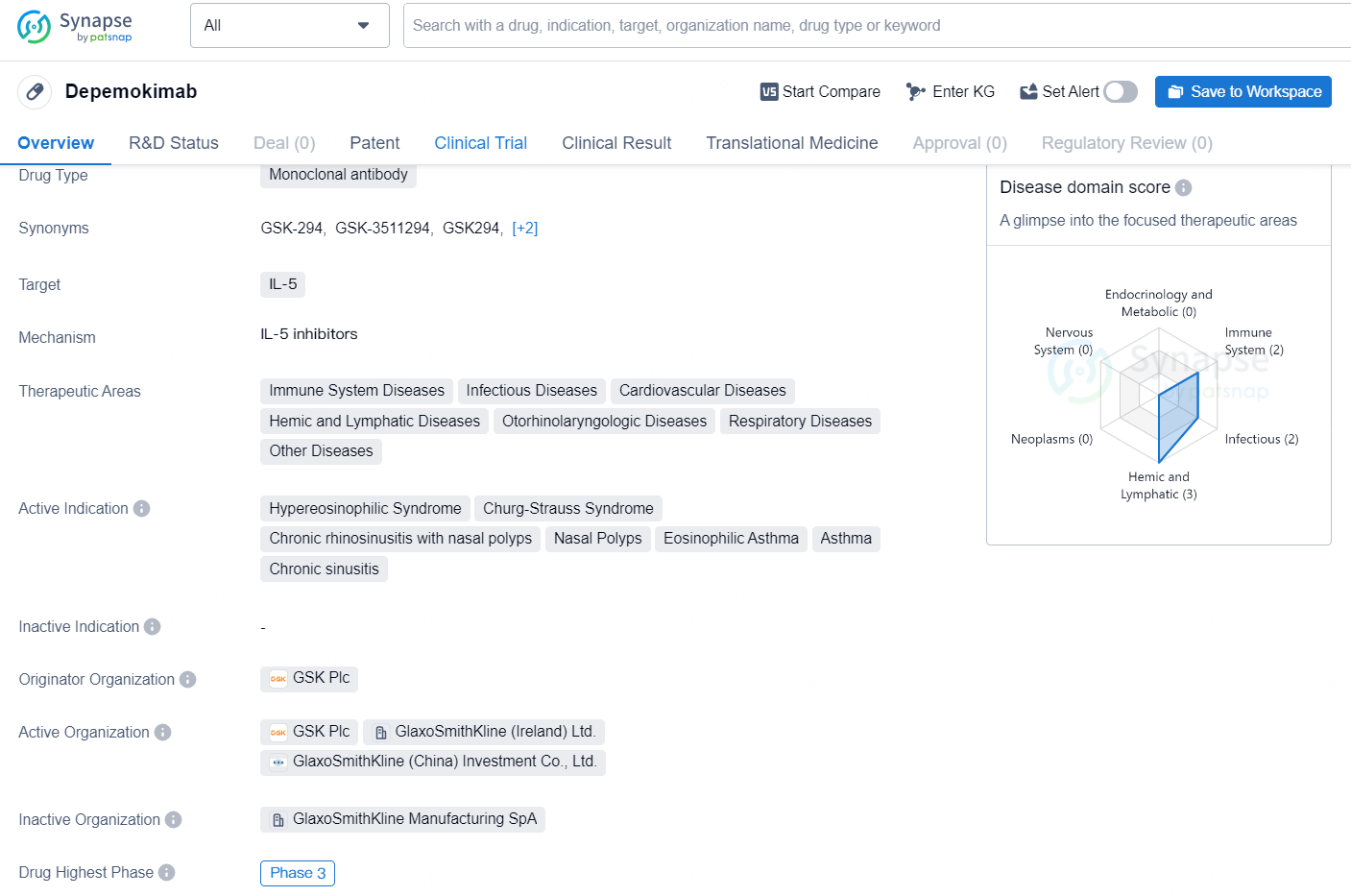
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Kaivan Khavandi, Senior Vice President and Global Head of Respiratory/Immunology Research & Development at GSK, stated: “Millions of individuals worldwide suffer from uncontrolled CRSwNP, with most showing signs of type 2 inflammation. These patients often have significant exposure to corticosteroids and frequently face the return of nasal polyps after undergoing surgery. We are encouraged by the findings from the ANCHOR studies, indicating that depemokimab may provide targeted and sustained inhibition of a crucial inflammatory pathway linked to the development of nasal polyps and nasal obstruction. The information shared today, along with recent phase III data concerning severe asthma, will be incorporated into regulatory submissions globally.”
Depemokimab represents the first ultra-long-acting biologic to undergo phase III testing, characterized by an extended half-life along with a strong binding affinity and efficacy for interleukin-5 (IL-5), potentially allowing for administration every six months for patients with CRSwNP. IL-5 is found in elevated amounts in nasal polyp tissue and plays a significant role as a cytokine in type 2 inflammation.
This data aligns with GSK's goals to improve treatment outcomes for individuals with conditions characterized by type 2 inflammation like CRSwNP. Achieving prolonged suppression of the inflammatory processes that drive the disease pathophysiology could provide substantial benefits for both patients and healthcare providers by diminishing the risk of inflammation recurrence resulting from missed doses. Lengthening dosing intervals may also lessen the necessity for frequent clinical visits.
CRSwNP is a chronic condition impacting up to 4% of the general public, with 40% experiencing uncontrolled symptoms. It arises from inflammation of the nasal lining, which can cause the formation of soft tissue growths known as nasal polyps. Those affected by CRSwNP may experience various symptoms, including nasal congestion, a reduced sense of smell, facial pressure, sleep disruptions, infections, and nasal discharge, all of which can significantly impact their emotional and physical health.
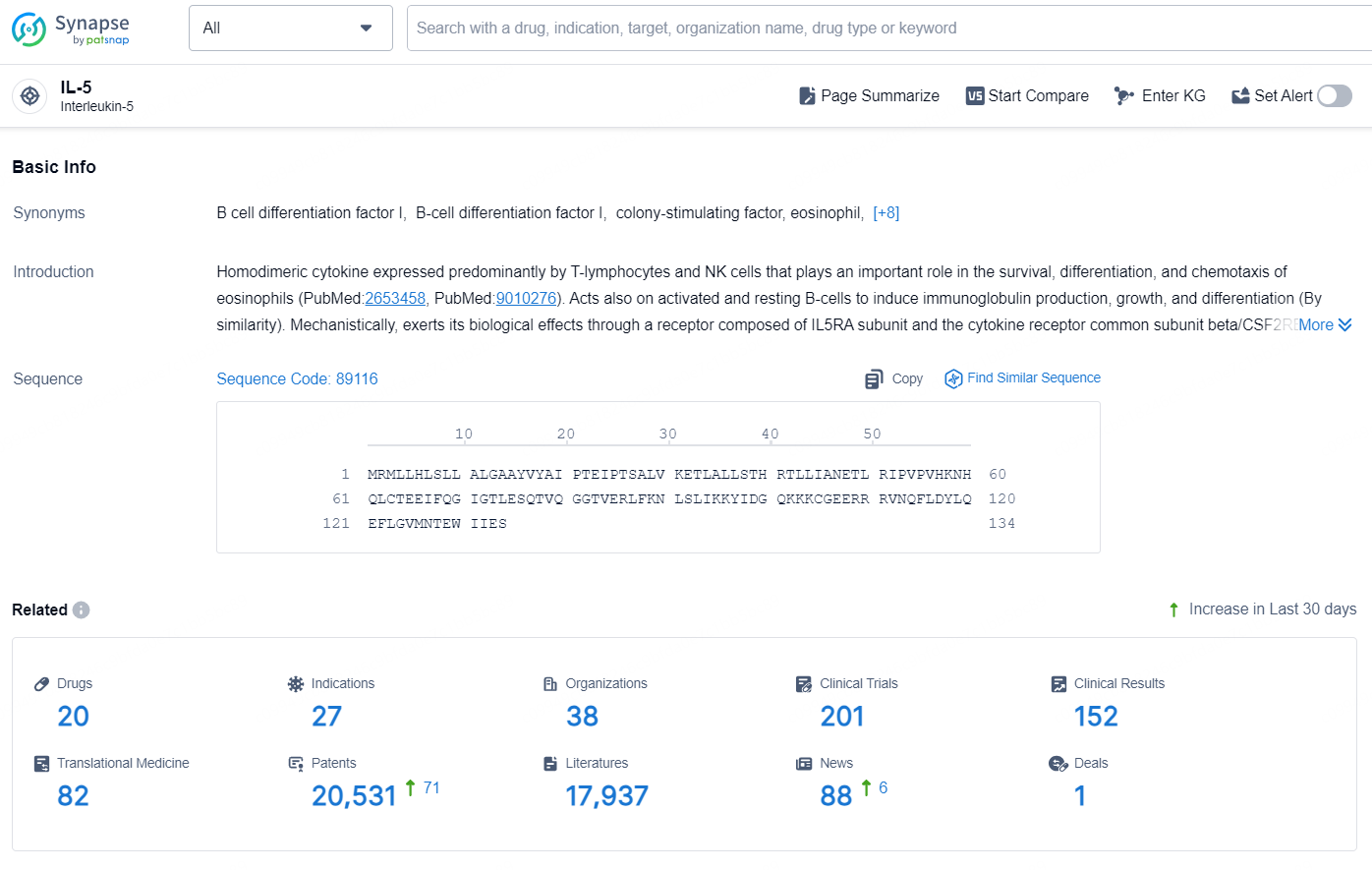
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 15, 2024, there are 20 investigational drugs for the IL-5 targets, including 27 indications, 38 R&D institutions involved, with related clinical trials reaching 201, and as many as 20531 patents.
Depemokimab is a monoclonal antibody drug that targets IL-5, a cytokine involved in the regulation of eosinophil production and function. Its therapeutic areas include immune system diseases, infectious diseases, cardiovascular diseases, hemic and lymphatic diseases, otorhinolaryngologic diseases, respiratory diseases, and other diseases. The drug's active indications are hypereosinophilic syndrome, Churg-Strauss syndrome, chronic rhinosinusitis with nasal polyps, nasal polyps, eosinophilic asthma, asthma, and chronic sinusitis.