Targeted Therapy for Multiple Myeloma: GPRC5D
A recent review in the journal Nature Reviews Clinical Oncology has explored new therapeutic approaches for multiple myeloma (MM) targeting cell membrane proteins. Among these are B-cell maturation antigen (BCMA), G-protein-coupled receptor family C group 5 member D (GPRC5D), and Fc receptor-like protein 5 (FcRL5). These are transmembrane proteins expressed on plasma cells and have been identified as prominent immunotherapeutic targets in MM over the past decade. They've shown significant activity in heavily pre-treated patients with relapsed and/or refractory disease.
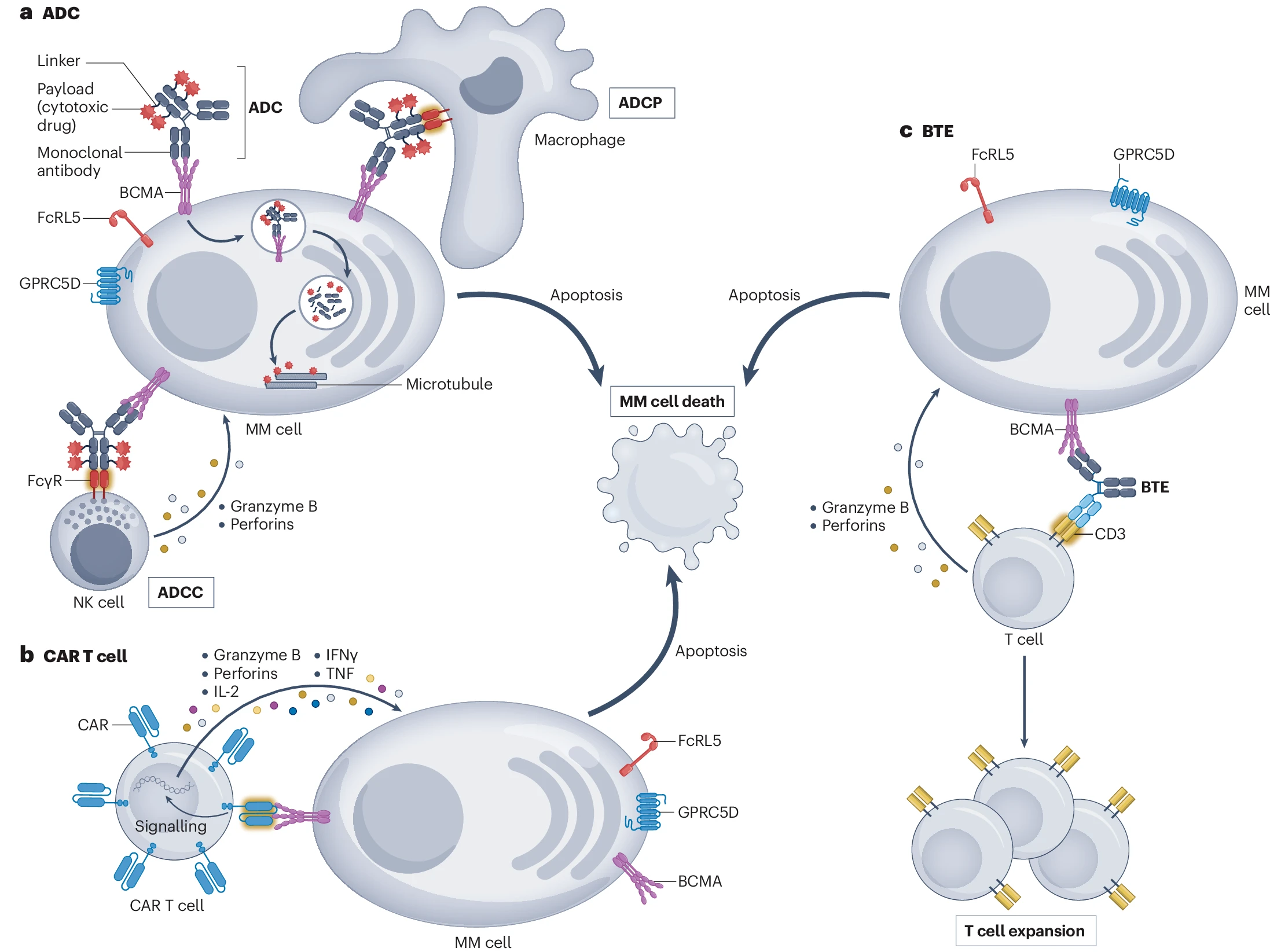
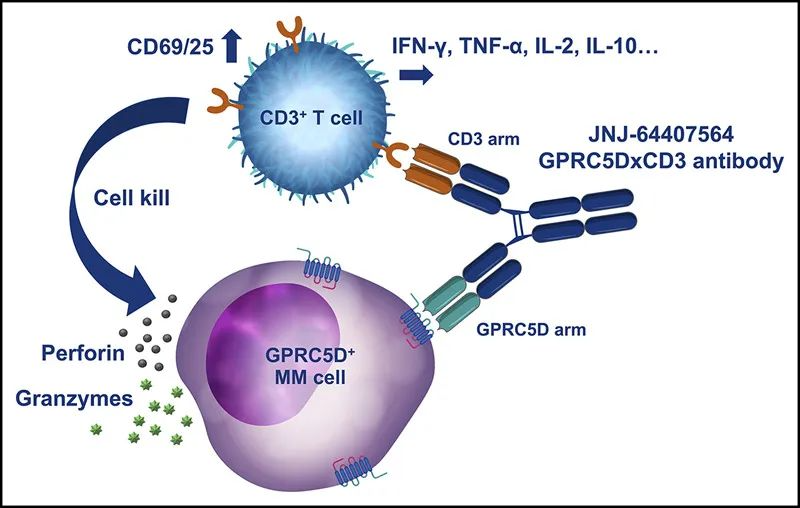
In August 2023, Johnson & Johnson announced that the U.S. FDA had approved TALVEY™ (talquetamab-tgvs) for the treatment of adult patients with relapsed or refractory multiple myeloma. Talquetamab is the first-in-class bispecific antibody that binds to GPRC5D, a protein highly expressed on multiple myeloma cells, and the CD3 molecule on the surface of T cells. This binding facilitates the recognition and attack of myeloma cells by T cells. The engagement activates T cells, leading to their proliferation and cytokine release, which triggers a cytotoxic response against the tumor cells.
GPRC5D (G-protein-coupled receptor family C group 5 member D) is a protein found on the surface of human cells, belonging to the GPCR family, and is classified as an orphan receptor whose natural ligand is yet to be identified. As of 2019, PubMed database searches revealed fewer than 20 publications on GPRC5D before then. How did Johnson & Johnson identify GPRC5D so quickly by 2016 and utilize it in developing the world's first bispecific product?
Preliminary Target Identification
In 2001, a team led by Danish scientist Hans Bräuner-Osborne published pioneering work on the cloning and identification of GPRC5D. This receptor is predicted to have seven transmembrane segments and is expressed on cell membranes. The target shows 31-42% amino acid sequence homology among the four human receptor subtypes in the C family GPCR's fifth group, but lower homology with the transmembrane domains of metabotropic glutamate receptor subtypes 2 and 3 and other C family members. GPRC5D was found on chromosome 12p13.3, located alongside RAIG1, and is expressed peripherally with unknown function.
Tissue Distribution
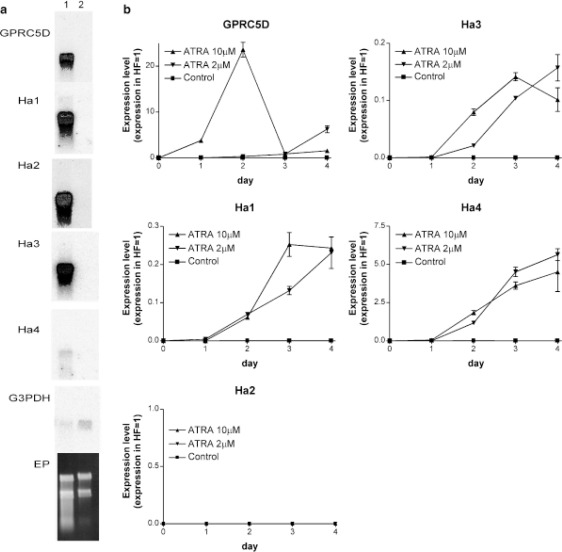
In 2004, a Japanese team led by Shinichi Inoue studied the unique tissue distribution and expression mechanism of GPRC5D. Their research demonstrated that GPRC5D is expressed in differentiated cells that produce hard keratin, including cortical cells of the hair shaft and keratinized regions of nails. GPRC5D transcripts are present during the mid-to-late anagen and catagen phases of hair growth but not during the telogen and early anagen phases. The differentiation inducer all-trans retinoic acid (ATRA) can induce GPRC5D expression in cultured hair follicle matrix cells. Therefore, GPRC5D's biological function is thought to play a critical role in hard keratinization.
ATRA, a derivative of vitamin A, plays important roles in regulating gene expression, promoting cell differentiation, and inhibiting cell proliferation. It has been extensively studied, especially in cancer treatment. RAIG1, an orphan GPCR member related to GPRC5D, was the first to be shown to be induced by ATRA in head and neck squamous cell carcinoma (HNSCC). Subsequently, related family members GPRC5B and GPRC5C have also been shown to have increased mRNA levels induced by ATRA treatment. The figure below provides evidence of GPRC5D expression induced by ATRA in cultured hair follicle matrix cells.
Genetic Evidence
Early genome-wide RNA interference (RNAi) screening experiments conducted in the breast tumor cell line MCF7 revealed a significant correlation between the knockdown of GPRC5D and resistance to the estrogen receptor modulator Tamoxifen in breast cancer cells. This correlation involved multiple tumor-related genes.
Clinical Relevance Studies
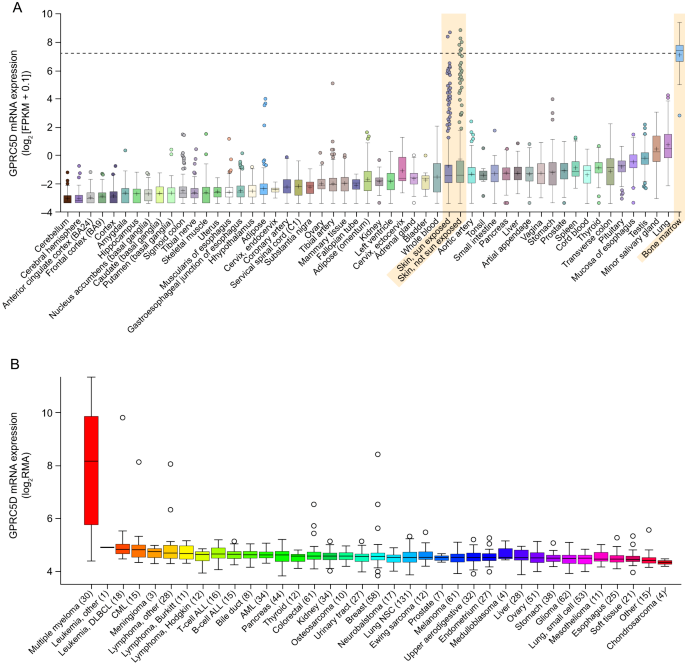
In 2012, a team led by Alexander Gaiger at the Medical University of Vienna in Austria analyzed GPRC5D mRNA expression levels in the bone marrow of 48 patients with multiple myeloma. Their findings indicated that GPRC5D expression is low in normal tissues but elevated in multiple myeloma. Furthermore, high GPRC5D expression was positively correlated with high plasma cell counts, elevated β2-microglobulin levels in the bone marrow, and various high-risk cytogenetic features. This suggests that GPRC5D may serve as a novel cancer antigen and therapeutic target.
The expression of GPRC5D could help distinguish high-risk from low-risk multiple myeloma patients, implying a role for GPRC5D in the pathogenesis of multiple myeloma and its potential clinical applications. Targeting GPRC5D could guide clinical treatment decisions and the development of anti-tumor drugs.
Concurrently, Israeli scientist Yossi Cohen and his team conducted clinical studies on multiple myeloma, demonstrating high levels of GPRC5D gene expression in bone marrow samples from 10 MM cases, while expression was low in 8 other hematological malignancies. This strong selective expression makes the GPRC5D gene and its encoded surface protein a reliable marker for monitoring tumor burden.
Further research observed that GPRC5D expression levels significantly decreased following anti-myeloma treatment. This decrease suggests that GPRC5D expression could serve as an indicator for evaluating treatment efficacy, and developing clinical therapeutic drugs targeting GPRC5D may provide novel treatment options for patients with high expression of this receptor.
Differential Expression Characteristics
The differential expression profile of GPRC5D in healthy tissues, MM patients, and various tumor cells is illustrated in Figure 4 . Comprehensive genetic, biological, and clinical evidence suggests that developing therapeutic drugs targeting GPRC5D may offer new opportunities for the treatment of MM patients.
Early focus of biological functions
Though the endogenous ligand for GPRC5D is unknown, and limited research has been conducted on its upstream and downstream biological functions, potential insights into its biological functions can be drawn from various dimensions before Johnson & Johnson selected GPRC5D for drug development in 2019.
Dimension 1 - Cancer: GPRC5A, a member of the GPCR subfamily to which GPRC5D belongs, has been studied in detail. Extensive research on GPRC5A suggests that the protein may play diverse roles in various cancer models. For instance, in non-small cell lung cancer (NSCLC) models, loss of GPRC5A activates NF-κB in mice, promoting lung inflammation and tumor formation, and enhances the transformed phenotype of normal and malignant lung epithelial cells through the STAT3 signaling pathway. In oral squamous cell carcinoma (OSCC) models, GPRC5A is highly expressed in normal oral tissues, particularly in differentiated areas. In vitro experiments demonstrate that overexpression of GPRC5A in OSCC CAL27 cells inhibits anchorage-independent growth, implying a tumor suppressor role for GPRC5A in oral tissues. The role of GPRC5D in MM tumor cells warrants further investigation.
Dimension 2 - Skin Health: Oral collagen peptides (CP) are generally considered beneficial for the skin. In vivo studies in mice have shown that oral collagen peptides increase the expression of proteins such as GPRC5D, keratin-associated protein (Krtap), and keratin (Krt) in mouse skin. These proteins play key roles in various aspects of skin health, including barrier function, hair structural integrity, and overall skin health. The relationship between GPRC5D and skin health merits further study.
Preclinical Model Validation
Between 2016 and 2018, companies such as Johnson & Johnson, Daiichi Sankyo, Roche, Chugai, and Juno Therapeutics submitted patents for GPRC5D antibody development or chimeric antigen receptors for oncology research. Preclinical model studies validated the therapeutic potential of targeting GPRC5D. For example, in 2016, Johnson & Johnson submitted a priority patent for bispecific antibodies targeting GPRC5D and CD3 (WO2018017786A2). These multispecific antibodies or antigen-binding fragments may be useful in treating GPRC5D-expressing cancers, particularly B-cell malignancies like multiple myeloma (MM).
Given the difficulty in preparing GPRC5D antigens, Johnson & Johnson opted to use HEK293 cells expressing GPRC5D as the antigen. They utilized their proprietary human Fab library for phage display screening, which included classical cell selection and FACS screening, and identified target antibody sequences using NGS sequencing. After developing the bispecific antibody, they conducted in vitro T-cell-dependent cytotoxicity assessments and in vivo efficacy evaluations.
In 2020, Johnson & Johnson published an article in the journal Blood detailing the development process of the bispecific antibody JNJ-64407564 and its preclinical study data in MM models. As demonstrated by the results, the bispecific antibody JNJ-64407564 effectively promoted T-cell-mediated cytotoxicity in various GPRC5D+ cells (MM.1R, OPM-2, H929 cell lines), while there was no effect on the control group, which consisted of GPRC5D- cell lines.
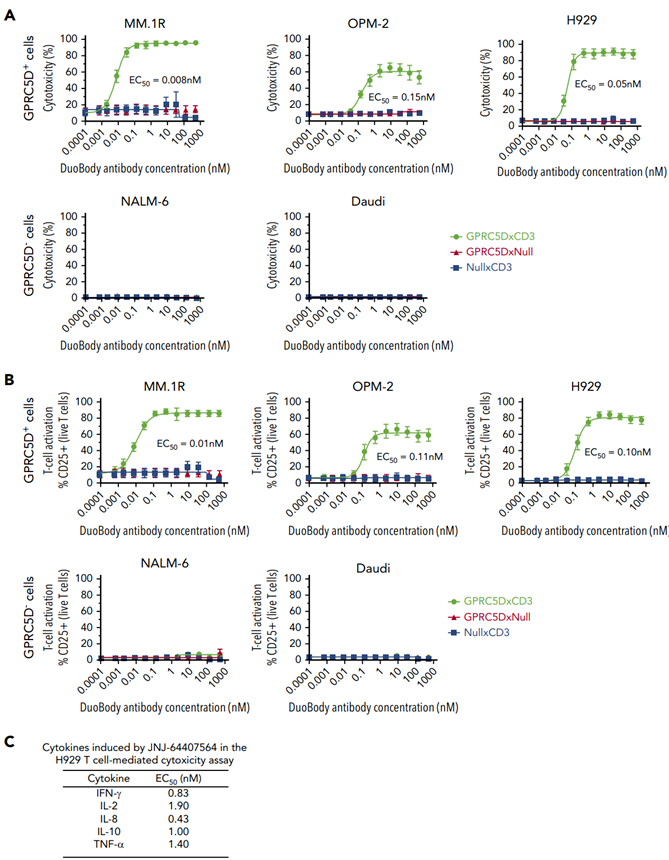
Regarding the GPRC5D x CD3-related antibody discovery, the Johnson & Johnson team has had 8 related patent applications approved and is conducting several clinical studies focused on MM and RRMM.
During the same period, Chugai released preclinical data on its bispecific T-cell redirecting antibody targeting GPRC5D/CD3, developed for the treatment of multiple myeloma.
Researchers established xenograft tumor models in NOD-SCID mice using the GPRC5D-expressing NCI-H929 (a human multiple myeloma cell line isolated by the NCI) and KMS-26 (a multiple myeloma cell line derived from a Japanese patient), and inoculated these mice with human T cells. The results showed that a single intravenous dose of 10 mg/kg of the GPRC5D/CD3 bispecific antibody significantly reduced the tumor volume of NCI-H929 and KMS-26 compared to a control antibody at the same dosage.
Researchers extracted total RNA from the tumors to analyze the expression of approximately 594 immune-related genes. Most genes upregulated by the GPRC5D/CD3 bispecific antibody were related to immune activation, such as IFNγ, IFNγ-induced chemokines CXCL9 and CXCL10, and IL2RA expressed on activated T cells. Collectively, these findings suggest that in mouse models, the GPRC5D/CD3 bispecific antibody exhibits potent antitumor activity against GPRC5D-expressing multiple myeloma by activating T cells.
Teams, including those at Novartis and Juno Therapeutics, pioneered the CAR (chimeric antigen receptor) strategy to develop cancer therapies targeting GPRC5D. In 2019, the Juno team reported in Science Translational Medicine on the world's first CAR-T cell therapy targeting GPRC5D. Using reporter cell lines capable of providing specific readouts of CAR signaling, researchers identified optimized CAR designs for linker length and reduced antigen-independent signaling, offering preclinical evidence that the CAR-T cell therapy candidate targeting GPRC5D is safe and effective.
Owing to this CAR-T product's leading position, Celgene acquired Juno for $9 billion in January 2018. The following year, Bristol-Myers Squibb completed its acquisition of Celgene for $74 billion, making Celgene a wholly-owned subsidiary of BMS. This acquisition strengthened BMS's standing in cancer treatment, particularly in immunotherapy and cell therapy. Through this merger, BMS gained multiple pipeline assets, including Juno Therapeutics, as well as the world's leading GPRC5D CAR-T pipeline.
Clinical Safety and Efficacy Studies
In December 2022, Johnson & Johnson published results in the New England Journal of Medicine from a Phase 1 clinical trial on the safety and efficacy of the T-cell redirecting GPRC5D bispecific antibody Talquetamab for multiple myeloma treatment. Results indicated that 232 patients received Talquetamab (102 intravenously, 130 subcutaneously). With median follow-up periods of 11.7 months for the 405 μg dose group and 4.2 months for the 800 μg dose group, patient response rates were 70% and 64%, respectively. The median response duration was 10.2 months and 7.8 months.
In terms of safety, the common adverse events for both subcutaneous dosing regimens (405 μg/kg weekly and 800 μg/kg biweekly) included cytokine release syndrome (noted in 77% and 80% of patients), skin-related events (67% and 70%), and oral ulcers (63% and 57%); apart from one instance of severe cytokine release syndrome, others were grades 1 or 2.
Based on subsequent submission materials to the FDA, Johnson & Johnson's Phase 2 MonumenTAL-1 study included patients who had received at least four prior therapies and were naive to T-cell redirecting therapies (187 patients), showing a significant overall response rate (ORR). At a 0.8 mg/kg SC biweekly dose, 73.6% of patients achieved ORR. After nearly 6 months of follow-up (range: 0 to 9.5 months), 58% achieved very good partial response (VGPR) or better, with 33% achieving complete response (CR) or better.
The most common grade 3/4 adverse events were hematological: anemia, neutropenia, lymphopenia, and thrombocytopenia, mostly during the initial treatment cycles. Common non-hematological adverse events included cytokine release syndrome, skin-related events, nail-related events, and taste disorders, with most dermatological adverse events being grades 1/2.
Subsequently, Roche developed the GPRC5D/CD3 bispecific antibody Forimtamig, which has demonstrated similar safety and efficacy profiles. In terms of efficacy, phase 1 clinical results for Forimtamig showed an objective response rate (ORR) of 71% in the intravenous (IV) cohort, with 59% of patients achieving very good partial response (VGPR) or better. The subcutaneous (SC) cohort reported an ORR of 64%, with 53% of patients reaching VGPR or better. The median time to the first response was 1.4 months and 1.6 months in the two cohorts. Regarding safety, dose reduction due to adverse events (AEs) occurred in 6 (12%) and 2 (4%) patients in the IV and SC cohorts, respectively; 3 (6%) and 5 (9%) patients discontinued the drug due to AEs. Cytokine release syndrome (CRS) was a common adverse event, usually low grade and confined to the first cycle, with a median onset time of 5 hours in the IV group and 24 hours in the SC group; most CRS events were resolved (98%). Hematologic adverse events were frequent in both groups. AEs related to non-myeloma cell GPRC5D expression were mostly grade 1/2, including skin-related events, mucosal events, and changes in hair and nails.
Compared with bispecific antibody products, several CAR-T products have shown better efficacy, albeit in a limited number of patients. For example, Juno's CAR-T product MCARH109 had a 71% ORR in the overall population (n=17); OriCell’s OriCAR-017 achieved a 100% ORR in the overall population (n=10); BMS's BMS-986393 reported a 100% ORR in its overall efficacy population (N=19).
Market Competition Landscape
As a representative GPCR target in recent years, GPRC5D is increasingly favored in the treatment of multiple myeloma (MM). To date, various bispecific (BCMA/GPRC5D, CD3/GPRC5D, CD38/GPRC5D) or trispecific products (BCMA/CD3/GPRC5D, BCMA/NKG2D/GPRC5D, BCMA/CD30/GPRC5D), as well as new modalities like CAR-T, CAR-NK, and ADC, have emerged rapidly, supported by advancing technologies.
Technological innovation continues to spur advancements in anti-cancer drug development across the industry. With the progress of cancer immunology, GPRC5D has become an important target in cancer immunotherapy due to its advantageous expression profile (low natural expression and high expression in MM tumors). While the potential clinical indications for GPRC5D are currently mainly limited to multiple myeloma, the long-standing unmet clinical needs in the MM space have become a crucial foundation for this target's rising popularity. Talquetamab from Johnson & Johnson, the first and only approved drug in this area, is expected to face competition from multiple entrants over the next 2-5 years, bringing real benefits to patients.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!