GSK reports promising phase III trial outcomes for Nucala (mepolizumab) in treating COPD
GSK plc (LSE/NYSE: GSK) released encouraging top-line outcomes from MATINEE, a phase III study assessing Nucala (mepolizumab), a monoclonal antibody aimed at interleukin-5 (IL-5), in adult patients suffering from chronic obstructive pulmonary disease (COPD).
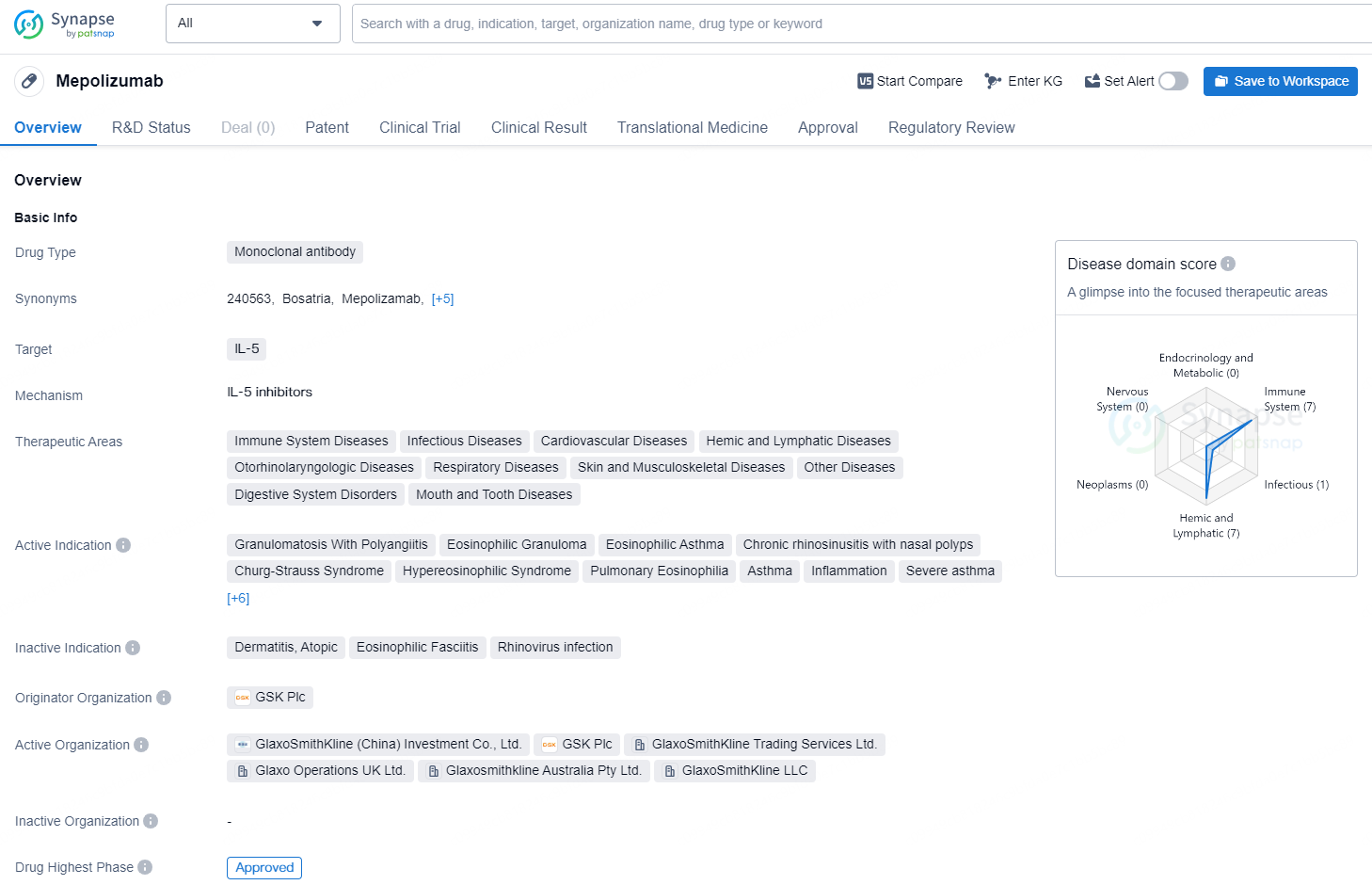
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The clinical trial enrolled individuals with COPD, characterized by symptoms of chronic bronchitis and/or emphysema, who were on optimized inhaled maintenance treatments. Participants needed to demonstrate type 2 inflammation marked by an elevated blood eosinophil count. The MATINEE trial achieved its primary goal by adding Nucala to the inhaled maintenance regimen, revealing a statistically significant and clinically significant decrease in the annual rate of moderate to severe exacerbations compared to a placebo in patients treated for up to 104 weeks.
Initial safety findings align with the established safety profile of Nucala. Additional analyses of the data are currently in progress.
COPD impacts over 300 million individuals worldwide, with up to 40% of patients showing type 2 inflammation indicated by a heightened blood eosinophil level, which contributes to exacerbations. IL-5 is a crucial cytokine in type 2 inflammation. Frequent exacerbations cause lung damage, a decline in lung function over time, and increased hospitalization risk. This cycle can lead to an overall deterioration in physical health, worsening of symptoms, reduced quality of life, and higher mortality.
The comprehensive results of MATINEE will be shared at an upcoming scientific conference and will support ongoing discussions with regulatory agencies. Currently, Nucala is not approved for treating COPD in any country.
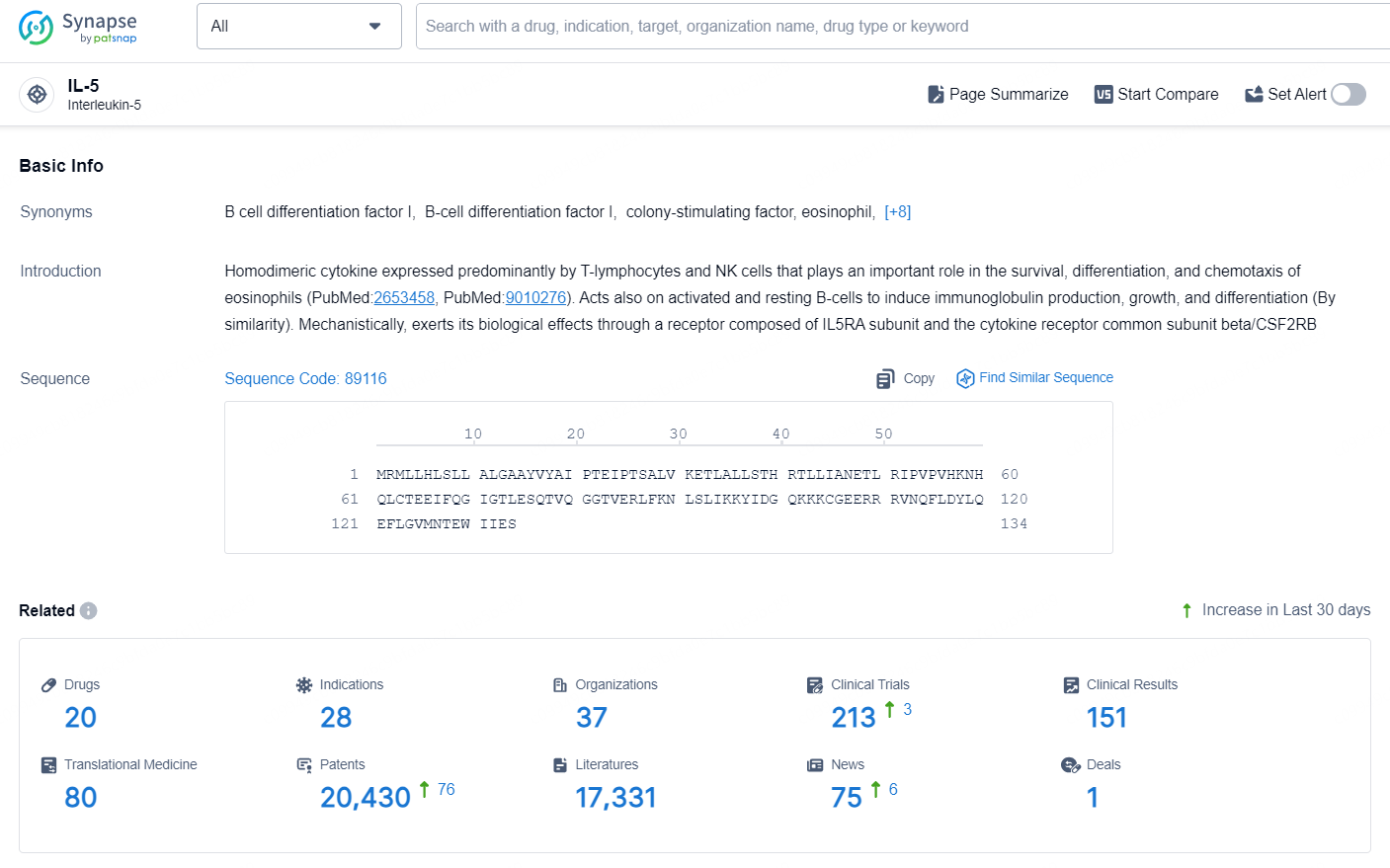
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 9, 2024, there are 20 investigational drugs for the IL-5 targets, including 28 indications, 37 R&D institutions involved, with related clinical trials reaching 213, and as many as 20430 patents.
Mepolizumab is a monoclonal antibody drug that targets IL-5 and is used in the treatment of various therapeutic areas including immune system diseases, infectious diseases, cardiovascular diseases, hemic and lymphatic diseases, otorhinolaryngologic diseases, respiratory diseases, skin and musculoskeletal diseases, other diseases, digestive system disorders, and mouth and tooth diseases. The active indications for Mepolizumab include granulomatosis with polyangiitis, eosinophilic granuloma, eosinophilic asthma, chronic rhinosinusitis with nasal polyps, Churg-Strauss syndrome, hypereosinophilic syndrome, pulmonary eosinophilia, asthma, inflammation, severe asthma, nasal polyps, pulmonary disease, chronic obstructive, eosinophilia, deglutition disorders, eosinophilic esophagitis, and chronic urticaria.