Halia Therapeutics Revels $30M Series C Funding for Inflammation Treatments
Halia Therapeutics, an emerging leader in the biopharmaceutical field focused on the development of new small molecule drugs to tackle inflammatory diseases, has successfully finalized its Series C funding round amounting to $30 million. The investment round was spearheaded by Todd Pedersen, and saw sustained contributions from prior backers.
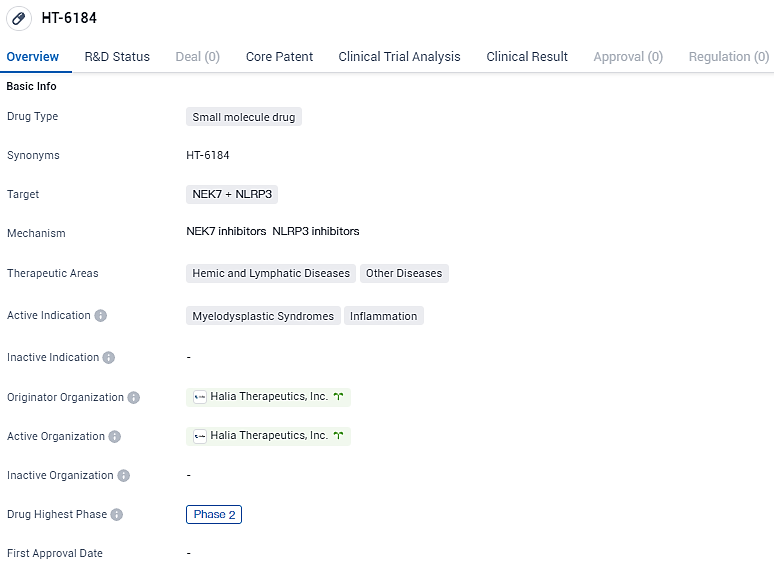
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The capital raised through this funding round is earmarked for the progression of Halia's principal compound, HT-6184. This compound is a first-of-its-kind, NLRP3/NEK7 inflammasome inhibitor, known for its selectivity and effectiveness when administered orally and is currently undergoing Phase II clinical evaluations. Halia Therapeutics is at the forefront of a new Phase IIa study in India, targeting therapy for individuals diagnosed with lower-risk myelodysplastic syndromes.
Lower-risk MDS refers to a spectrum of hematological malignancies characterized by defective and morphologically atypical bone marrow cells, identified as dysplastic. Moreover, the company is intent on initiating Phase II U.S. trials for HT-6184 to explore its efficacy in controlling inflammatory pain following medical procedures, and embarking on a Phase I study in those affected by Alzheimer's disease.
The investment will further facilitate pre-INVESTIGATIONAL New Drug activities for Halia's nascent projects, which focus on the development of Leucine-rich repeat kinase 2 (LRRK2) antagonists. These are being researched as potential treatments for neurodegenerative disorders like Parkinson's and Alzheimer's disease. Resources are also allocated to bolster both the clinical and regulatory teams, substantially strengthening the company’s capacity to drive its portfolio's international progression.
Todd Pedersen reflects on the innovation of Halia Therapeutics, stating, "With Halia's pioneering strategy in honing in on the NLP3 inflammasome, we anticipate groundbreaking progress in the treatment of diverse inflammatory conditions, blood disorders, and various cancers." He further underscores the invaluable contributions of the company, highlighting its expertise and commitment to patient-centric development as critical in transforming the management of chronic inflammation for affected individuals.
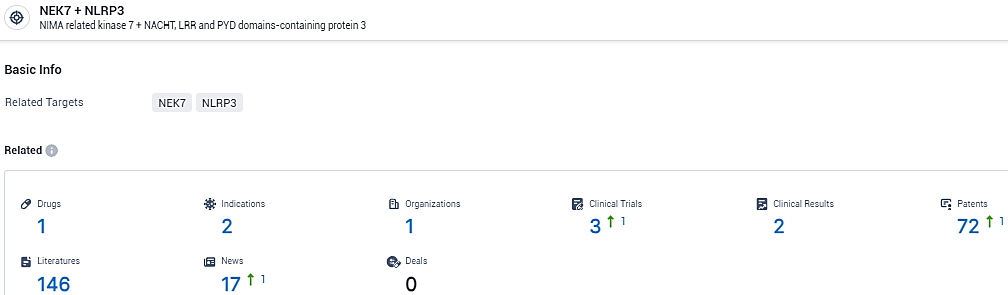
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 8, 2024, there are 1 investigational drugs for the NLRP3/NEK7 target, including 2indications, 1R&D institutions involved, with related clinical trials reaching 3, and as many as 72 patents.
HT-6184 shows promise as a potential treatment for myelodysplastic syndromes and inflammation. Its targeting of NEK7 and NLRP3 highlights its potential to modulate disease pathways and provide therapeutic benefits. With its current status in Phase 2, further clinical trials will be necessary to evaluate its safety and efficacy in larger patient populations. If successful, HT-6184 could address unmet medical needs in the field of hemic and lymphatic diseases, as well as other diseases associated with inflammation.