New Insights: Vabysmo Effectively Treats Retinal Swelling in Retinal Vein Occlusion Patients
Genentech, which is part of the Roche Group, disclosed fresh insights spanning 72 weeks from two international Phase III trials, named BALATON and COMINO. These studies were conducted to assess the efficacy of Vabysmo® (faricimab-svoa) for treating macular edema caused by branch retinal vein occlusion (BRVO) and central retinal vein occlusion (CRVO).
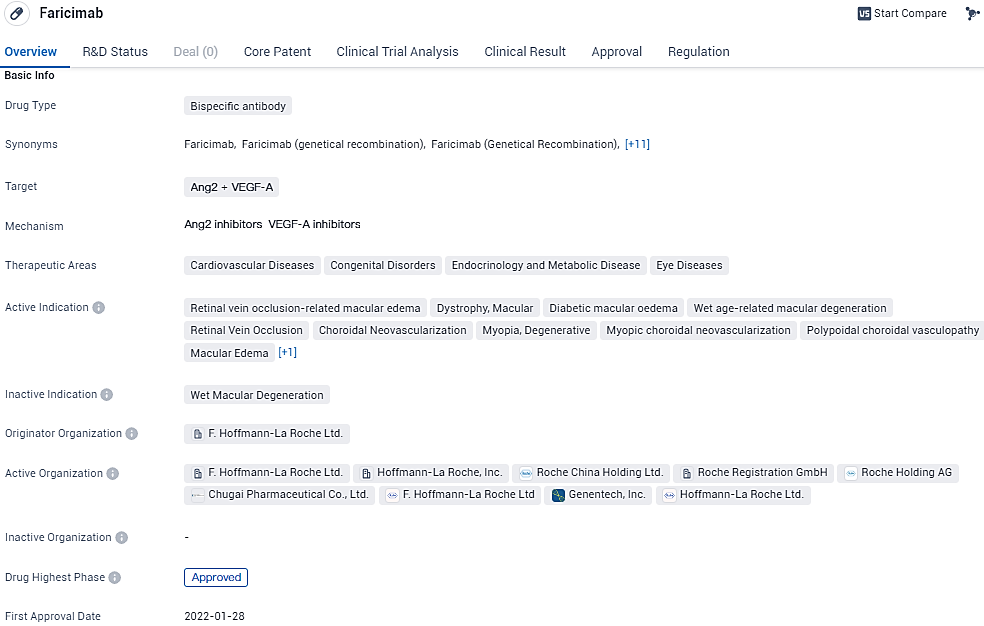
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Standard treatments for retinal vein occlusion (RVO) are usually administered every one to two months, but recent data indicates that Vabysmo, as used in the BALATON and COMINO trials, enabled approximately 60% and up to 48% of participants, respectively, to prolong the spacing of their treatment to every three to four months. Furthermore, participants in these trials managed to preserve their initial improvements in vision and significant reduction in retinal swelling for over one year, beyond the initial 24 weeks of observation.
Controlling retinal swelling is crucial because the accumulation of excessive fluid at the rear of the eye is linked to visual distortion and haziness. Vabysmo demonstrated a favorable safety record and was generally well-received in both trials, aligning with outcomes from prior research.
Levi Garraway, M.D., Ph.D., the Chief Medical Officer and Head of Global Product Development at Genentech, remarked that for the first time, global Phase III trials have reported the preservation of enhanced vision and anatomical benefits for over a year for patients with both branch and central RVO. According to Garraway, the extended-term findings complement the existing clinical evidence supporting the efficacy of Vabysmo as a treatment for retinal diseases that can impair vision.
Echoing this, Ramin Tadayoni, M.D., Ph.D., the Ophthalmology Department Head at Université Paris Cité and EURETINA President, emphasized the durability of vision advancement and retinal drying up to 72 weeks with Vabysmo as a therapeutic agent for RVO. He highlighted the necessity for more treatment options to better accommodate individuals with this disease, noting that Vabysmo could offer improved patient outcomes and fewer required visits to the clinic.
Vabysmo holds the distinction of being the inaugural bispecific antibody for ocular use to receive regulatory approval. It is engineered to counteract the pathways of Ang-2 and VEGF-A, two factors believed to play roles in vision impairment by causing blood vessel instability and contributing to the formation of new, prone-to-leakage vessels, as well as escalating inflammation. Ongoing studies aim to uncover more about how the Ang-2 pathway influences retinal diseases.
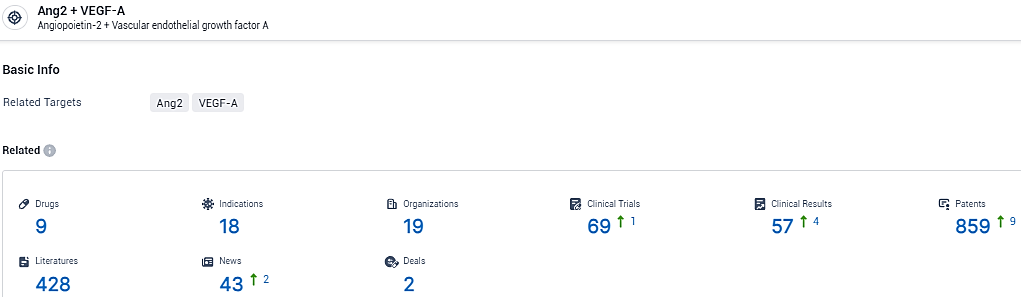
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 7, 2024, there are 9 investigational drugs for the Ang-2 and VEGF-A target, including 18 indications, 19 R&D institutions involved, with related clinical trials reaching 69, and as many as 859 patents.
Faricimab's approval and regulatory status, coupled with its unique bispecific antibody mechanism of action, position it as a promising therapeutic option in the field of biomedicine. Its potential to address a wide range of conditions and its approval in major markets signify its potential impact on patient care and the pharmaceutical industry as a whole.