Bio-Thera Solutions Initiates Phase 1B/2A Study for BAT6026 Monoclonal Antibody in Eczema Treatment
Bio-Thera Solutions, an enterprise currently marketing biopharmaceuticals while also focusing on advancing a range of novel treatment options and biosimilar products, has just declared the initiation of patient treatment in a combined Phase 1A/2B clinical study. This research is evaluating both the effectiveness and the risk profile of the compound BAT6026 in individuals who are experiencing atopic dermatitis ranging from moderate to severe intensity.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
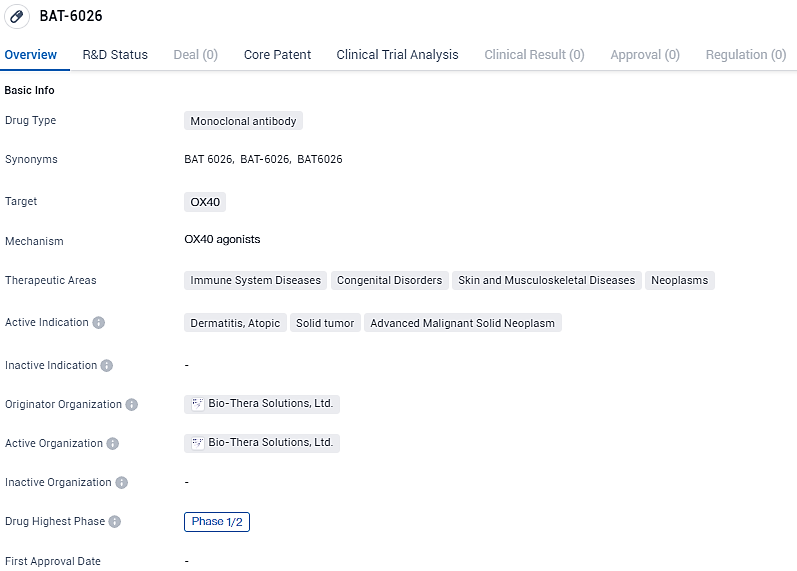
OX40 serves as a crucial target for drugs addressing autoimmune and inflammation concerns, noted for its elevated presence on CD4+ and CD8+ T cells when activated. These T cells are central to the pathology of various inflammatory conditions, including eczema. BAT6026 operates by inhibiting the interaction between OX40 and OX40L, curbing the activation and growth of T cells, while simultaneously targeting and reducing the number of active OX40+ T cells via an augmented antibody-dependent cellular cytotoxicity (ADCC) mechanism.
As a result of its mechanisms of action, BAT6026 is forecasted to provide treatment options for a range of inflammatory disorders, particularly those that involve Th2 cell activity. BAT6026 boasts a promising preclinical profile and has demonstrated safety and tolerability in a Phase 1 trial that incrementally increased doses.
The ongoing Phase IA/IIB, which is a multicenter study of BAT6026, is set to evaluate the therapeutic benefit and safety profile in individuals with atopic dermatitis ranging from moderate to severe. The research aims to ascertain the treatment's safety, tolerability, pharmacokinetic properties, and initial efficacy in reducing inflammation.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
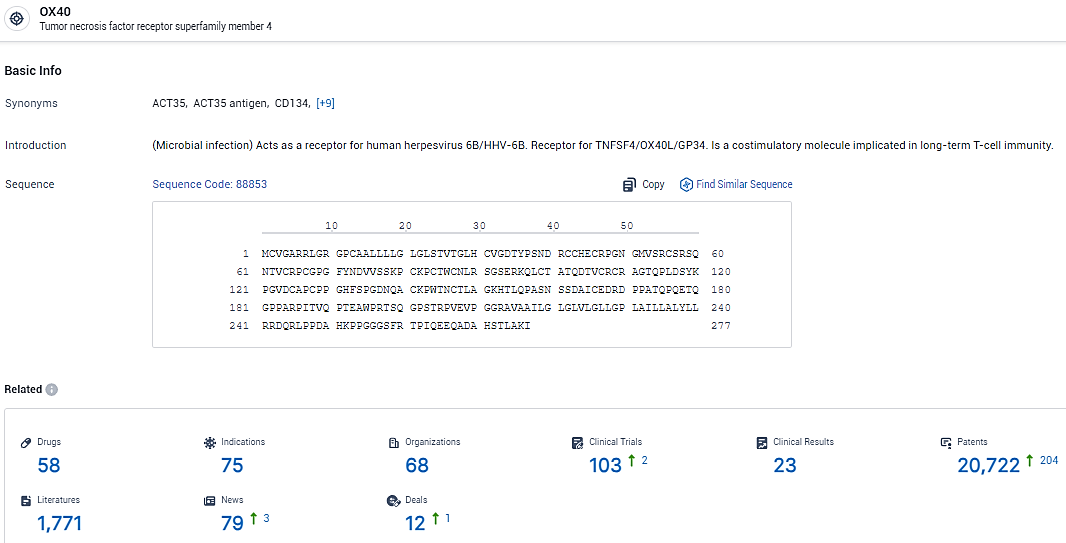
According to the data provided by the Synapse Database, As of February 7, 2024, there are 58 investigational drugs for the OX40 target, including 75 indications, 68 R&D institutions involved, with related clinical trials reaching 103, and as many as 20722 patents.
BAT-6026 targets OX40 and is being developed for the treatment of immune system diseases, congenital disorders, skin and musculoskeletal diseases, and neoplasms. The drug is currently in Phase 1/2 of development globally and in China.