Hanmi collaborates clinically and signs supply deal with MSD to test BH3120 along with KEYTRUDA® (pembrolizumab)
Hanmi Pharmaceutical, a prominent Korean biopharma corporation that concentrates on notable research fields, including oncology, obesity/metabolism, and rare disorders, has declared the formation of a Clinical Trial Collaboration and Supply Agreement with MSD.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
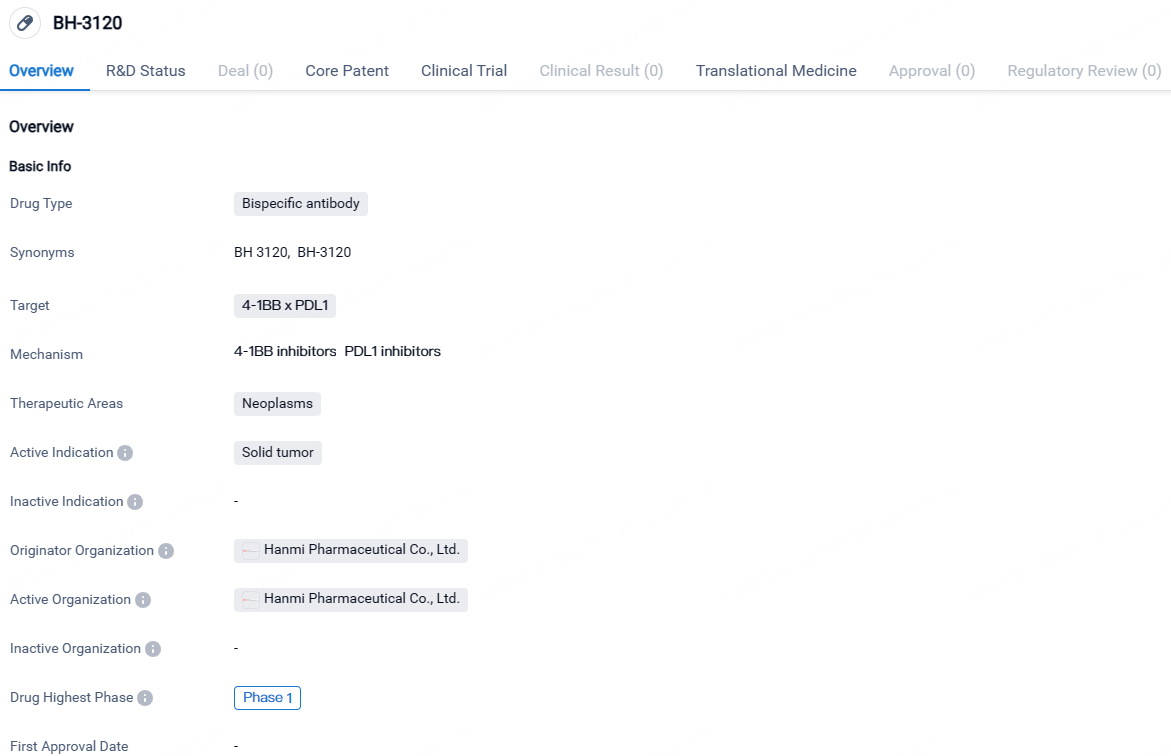
Following the signing of the agreement, Hanmi Pharmaceutical is set to initiate a phase 1 clinical trial to test the safety and efficacy of its cancer immunotherapy agent, ‘BH3120’, used together with MSD's anti-PD-1 therapy, KEYTRUDA® (pembrolizumab). This trial will focus on patients suffering from progressive or metastatic solid tumors. Hanmi Pharmaceutical will be the trial sponsor while MSD will provide KEYTRUDA.
The investigational drug ‘BH3120’ incorporates 'Pentambody', an advanced bispecific antibody technology, currently being co-developed by Hanmi Pharmaceutical and its subsidiary in China, BJHM.
Pentambody technology uniquely links one antibody to two different targets at the same time, which enhances the performance in both cancer immunotherapy and targeted therapy. Specifically, BH3120 functions as an IgG-like bivalent bispecific antibody, primarily targeting PD-L1 and 4-1BB, displaying a preference in binding affinity for PD-L1.
Other existing antibody therapies targeting 4-1BB often encounter issues with safety. However, diverse non-clinical trials have demonstrated that BH3120 can distinctly separate immune responses in the tumor microenvironment (TME) from those in normal tissues, highlighting its potential as both an effective and safe treatment for cancer.
Dr. Kim Dong-wan, who leads the Clinical Trials Center at Seoul National University Hospital, serves as the Principal Investigator for the phase 1 trial of BH3120 in both Korea and the US. He commented on the collaboration with MSD, saying, "With our joint efforts on BH3120 alongside KEYTRUDA, we aim to enhance treatment results for patients facing relapsed or refractory diseases."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
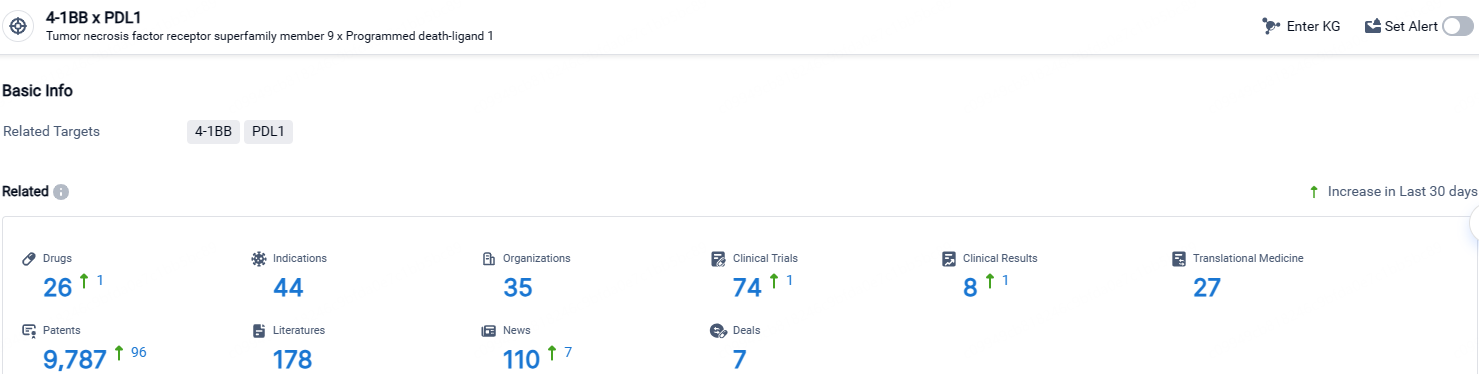
According to the data provided by the Synapse Database, As of April 25, 2024, there are 26 investigational drugs for the 4-1BB and PDL1 target, including 44 indications, 35 R&D institutions involved, with related clinical trials reaching 74, and as many as 9787 patents.
BH-3120 targets both 4-1BB and PDL1 and is being developed for the treatment of solid tumors. Currently in Phase 1, BH-3120 is undergoing early-stage clinical trials to assess its safety and efficacy.