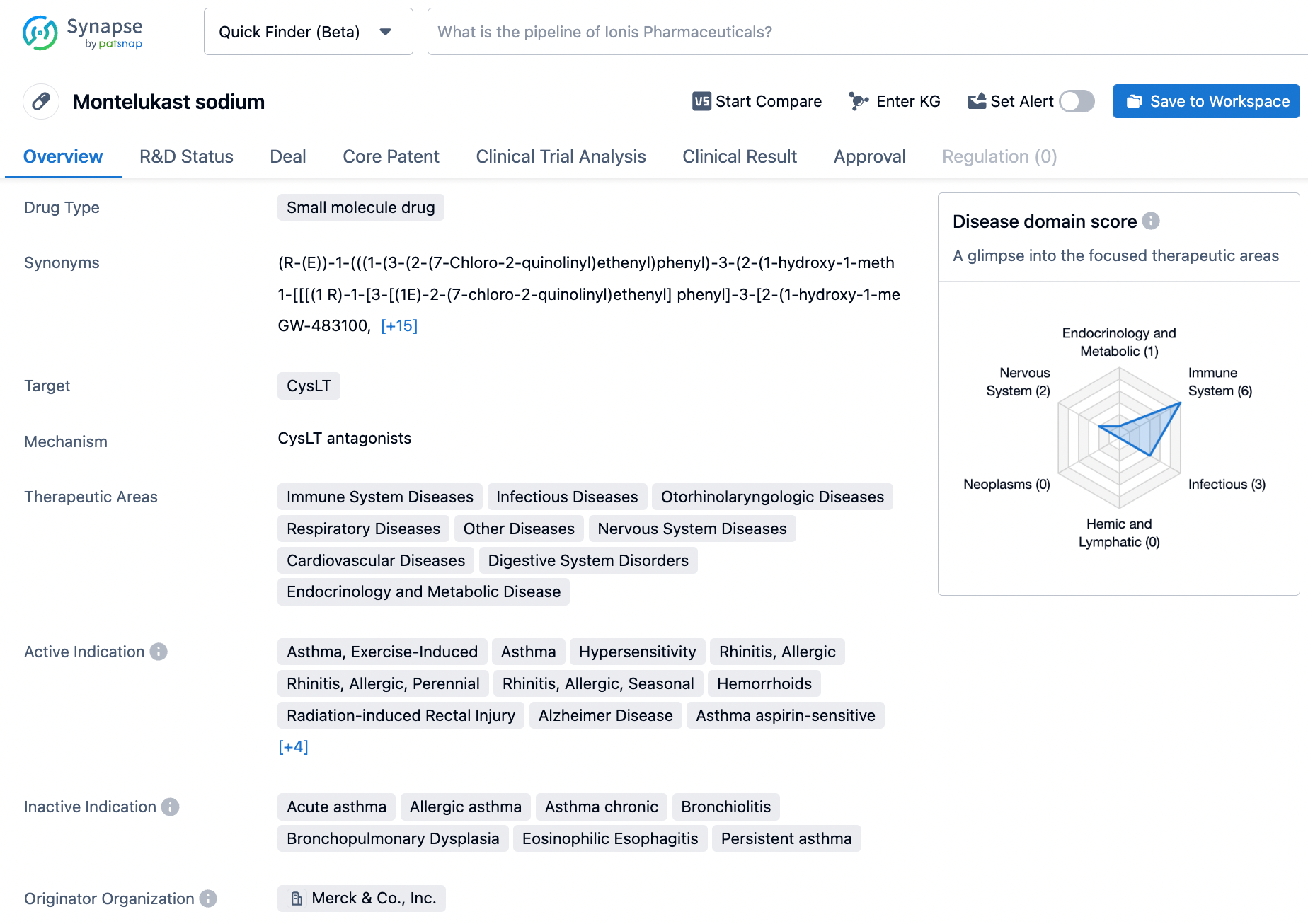
Harnessing Synapse for Montelukast Information: Strategies and Tips
Montelukast sodium, marketed under the trade name SINGULAIR®, is a medication approved for the treatment of asthma, exercise-induced bronchoconstriction (EIB), and allergic rhinitis. It belongs to the class of drugs known as CysLT antagonists and works by blocking leukotrienes, which are chemicals that cause inflammation in the body. Montelukast sodium was first approved in 1997 in Mexico and has since been widely used in the management of asthma and allergic rhinitis worldwide. Chemically described as [R-(E)]-1-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic acid, monosodium salt, the drug is available in tablet, chewable tablet, and oral granule formulations. Click on the image below to begin the exploration journey of Montelukast through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!