Heidelberg Pharma Shares Progress Report for Phase I/IIa Clinical Study Involving HDP-101
Heidelberg Pharma AG communicated that it has commenced patient recruitment for the fifth cohort in the Phase I/IIa clinical study of its primary developmental candidate HDP-101, operating with a dosage of 100 µg/kg.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The Patient Safety Review Board has assessed patient data and found that there have been no cases of dose-limiting toxicity up to now. Safety and tolerance have been demonstrated at the initial four dosage levels. To date, a total of 12 patients have received treatment during the trial.
Patient recruitment saw a substantial boost with the increased number of testing sites this summer. One patient from the third group has been undergoing HDP-101 monotherapy for more than nine months, having received eleven doses in total.
Dr. András Strassz, the Chief Medical Officer at Heidelberg Pharma remarked: “We are thrilled as our premier Amanitin-based antibody drug conjugate, HDP-101, is proving to be non-toxic and well-tolerated. We are set to proceed with dose enhancement as scheduled with our fifth group.”
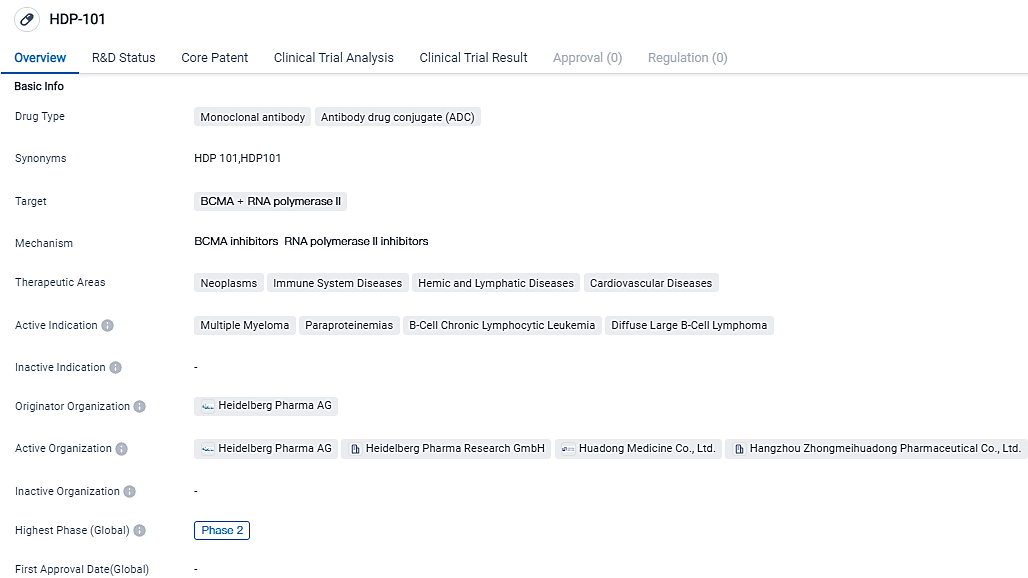
HDP-101, the BCMA antibody and Amanitin conjugate, is currently under trial in an unrestricted, multi-center study targeting the treatment of recurrent or resistant multiple myeloma, a malignant bone marrow disorder.
The preliminary segment of the trial is a Phase I dose enhancement study that aims to establish the highest tolerable dosage of HDP-101. These results will help set the dose for the Phase IIa segment, whose primary intent is to assess the anti-cancer efficacy of HDP-101 initially.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
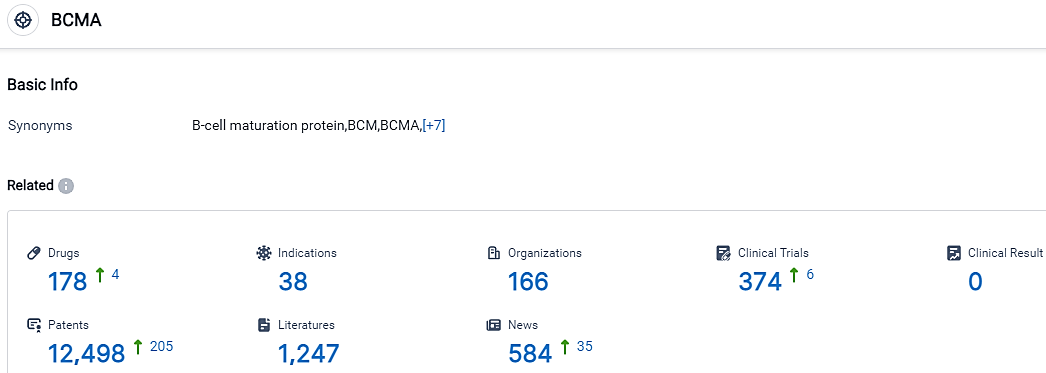
According to the data provided by the Synapse Database, As of September 20, 2023, there are 178 investigational drugs for the BCMA target, including 38 indications,166 R&D institutions involved, with related clinical trials reaching 374,and as many as 12498 patents.
HDP-101 is a drug in the field of biomedicine that falls under the category of Monoclonal antibody and ADC. It targets BCMA and RNA polymerase II. The drug is primarily focused on treating neoplasms, immune system diseases, hemic and lymphatic diseases, and cardiovascular diseases. It is important to note that this summary is based solely on the given information and does not include any subjective interpretations or fictional data.