Orexo submits NDA for OX124 to FDA, a high-potency antidote for opioid overdose
Orexo AB has recently reported that it has sent its NDA to the US FDA concerning OX124, which is a high-dose counteractive treatment intended for opioid overdoses and is administered nasally. OX124 is developed by leveraging Orexo's top-tier drug administration platform - amorphOX®, and it is crafted to mitigate the impacts of intense synthetic opioids, like fentanyl.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Findings from the critical OX124-002 trial in healthy volunteers support the submission, demonstrating that OX124 significantly enhances and accelerates the absorption of naloxone in contrast to an injection reference product delivered intramuscularly.
Moreover, previous exploratory clinical research has shown that the development formulations of OX124 offer superior bioavailability and quicker absorption than the current top-selling naloxone emergency medication, even at equivalent dosage levels. OX124's patent safeguards it until 2039.
During the latest 12-month period, ending April 2023, the projected annual lethal overdose cases in the US surpassed 110,000; 76 percent of these were tied to opioid overdoses, with 91 percent involving man-made opioid.
Earlier in the year, the FDA requested a new NDA due to difficulties with the packaging process, which have now been addressed in collaboration with the contract manufacturer. To guarantee the highest quality production, successful tests and certifications have been carried out at the contract manufacturer's facility.
Nikolaj Sørensen, President, and CEO of Orexo AB, expressed, "Due to the superb efforts of our team, OX124's supply chain now satisfies the most stringent reliability requirements, enabling us to promptly present a new NDA to the FDA. My team and I anticipate widening the reach of this unique and crucial medication." The FDA's evaluation process is set to last between ten and thirteen months. Orexo is preparing to roll out in the US in late 2024 or early 2025.
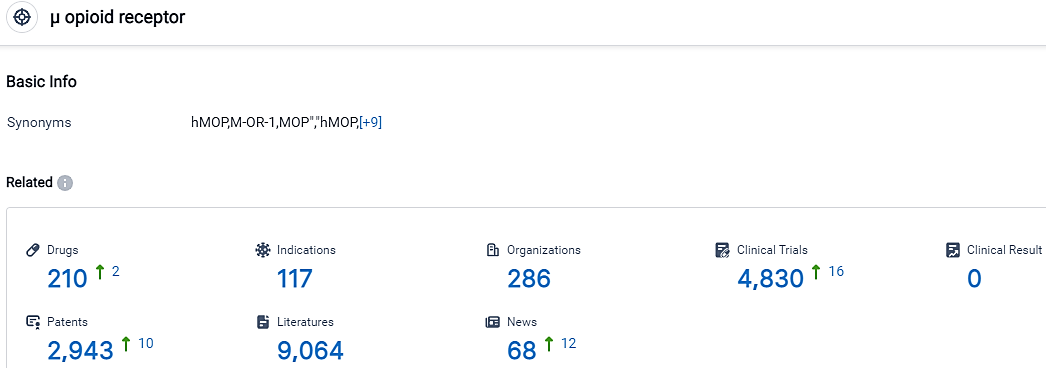
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 20, 2023, there are 210 investigational drugs for the μ opioid receptor, including 117 indications,286 R&D institutions involved, with related clinical trials reaching 4830,and as many as 2943 patents.
OX124 is a well-established small molecule drug that targets the μ opioid receptor and is primarily used for opiate overdose. With the submission of OX124 we are making good progress in expanding our commercial product portfolio in the US and this high-dose life-saving medication has the potential to curb the huge upturn in lethal overdoses seen over the past years and which is grounded in the wide spread of fentanyl.