HLX14 Biosimilar Meets Goals in Phase 3 Trial Against Prolia® and Xgeva®
Shanghai Henlius Biotech, Inc. alongside Organon has publicized the successful advancement of its Phase III comparative clinical study concerning the investigational biosimilar HLX14, patterned after Prolia/Xgeva (denosumab), which achieved its primary endpoints. The year prior, in 2022, Henlius finalized a licensing and supply contract granting Organon the exclusive rights to market two of its biosimilar candidates, one of them being HLX14. This contract encompasses territories including the United States, European Union, and Canada, with the notable exclusion of the Chinese market.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
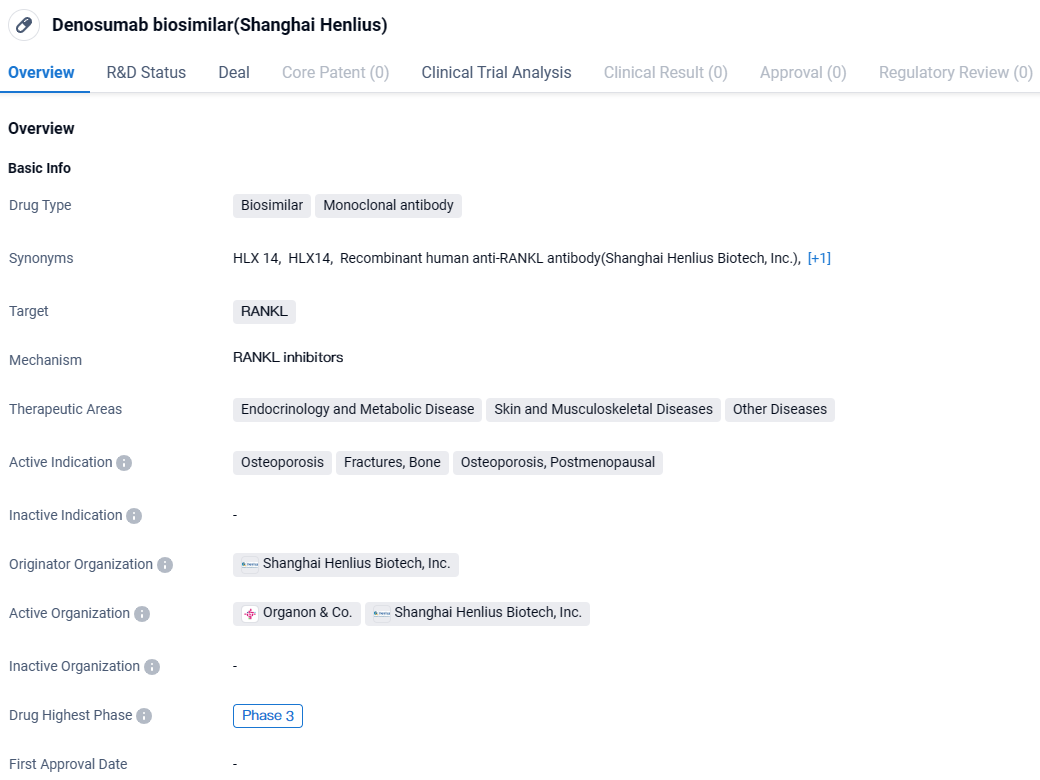
An international, multicenter, randomized, double-masked, parallel-group phase 3 trial was conducted to assess the effectiveness, safety profile, tolerability, and immune response of HLX14 compared to the European Union-approved reference drug denosumab (marketed as Prolia) among postmenopausal females at elevated fracture risk due to osteoporosis.
Participants fitting the study criteria were assigned in equal proportions to receive a 60 mg subcutaneous dose of either HLX14 or the reference medication denosumab (Prolia) biannually. The study's principal measure of effectiveness was the change percentage in the bone mineral density at the lumbar spine, from the starting point to Week 52 as determined by centralized radiologic evaluations. The main pharmacodynamic measure involved calculating the effect–time curve's area for the percentage fluctuation in serum biomarker type I collagen C-telopeptide levels from the study's onset to Week 26. The definitive goals set for this study were successfully achieved.
Denosumab is commercially available under various branding names across different nations and regions, indicated for multiple conditions, including as a therapeutic option for postmenopausal osteoporosis in women who are at a significant risk of breaking bones, among other uses.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
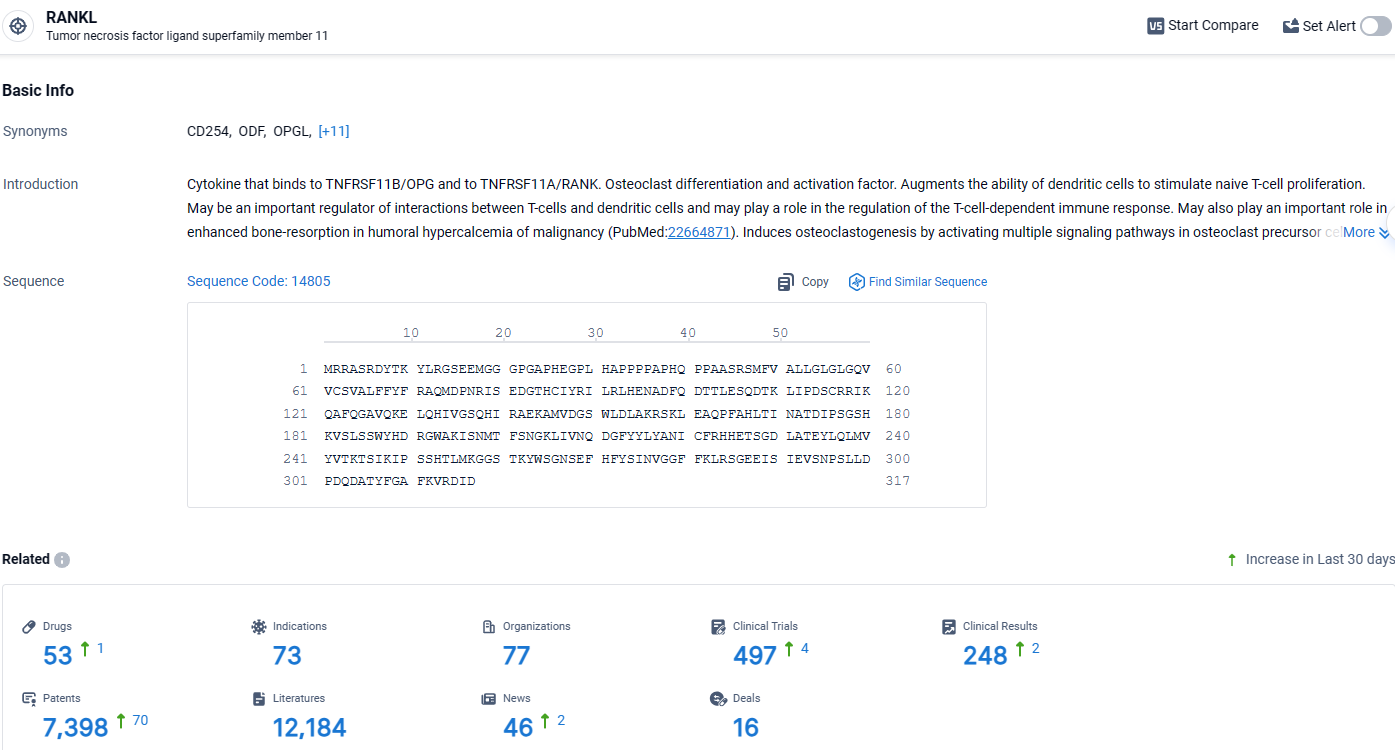
According to the data provided by the Synapse Database, As of April 9, 2024, there are 53 investigational drugs for the RANKL target, including 73 indications, 497 R&D institutions involved, with related clinical trials reaching 497, and as many as 248 patents.
HLX14 is a monoclonal antibody drug targeting RANKL. It shows promise in treating osteoporosis, fractures, and bone-related complications, particularly in postmenopausal women. With its highest phase of development being Phase 3, Denosumab biosimilar is advancing towards potential regulatory approval, offering a potential alternative treatment option in the field of biomedicine.