How does AstraZeneca's PARP inhibitor differentiate?
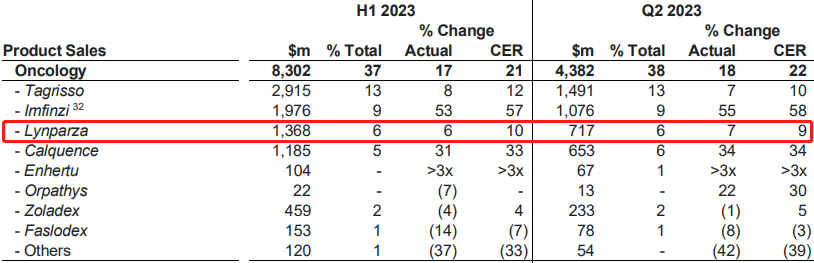
At the end of July, AstraZeneca released its semi-annual financial report for 2023. Eight of its drugs had sales exceeding one billion US dollars, among which Lynparza generated a revenue of $1.368 billion, a 10% increase from the first quarter and a 5.9% increase from the same period in 2022. The compound patent for Lynparza will expire in March next year, and the use patent will also expire in November next year.
Faced with this fiercely competitive PARP inhibitor battlefield, how will AstraZeneca respond?
Expansion of Olaparib’s New Indications and Combination Therapy
Firstly, there is the expansion of new indications and combination therapy with Olaparib. As shown in the Phase III clinical trial NCT02032823, Olaparib is used as an adjunctive treatment for high-risk HER2-negative primary breast cancer patients with a BRCA mutation lineage. Patients will be randomly assigned to the Olaparib group or placebo group at a 1:1 ratio. Random grouping will be stratified based on hormone receptor status, prior neoadjuvant chemotherapy vs adjuvant chemotherapy, and prior platinum-based drug treatment of breast cancer. Enrolling 1836 patients, patients will be orally administered Olaparib at a dose of 300mg BID. The primary endpoint is invasive disease-free survival (IDFS); secondary endpoints include distant disease-free survival (DDFS) and overall survival. Data shows that the primary endpoint has been reached at present.
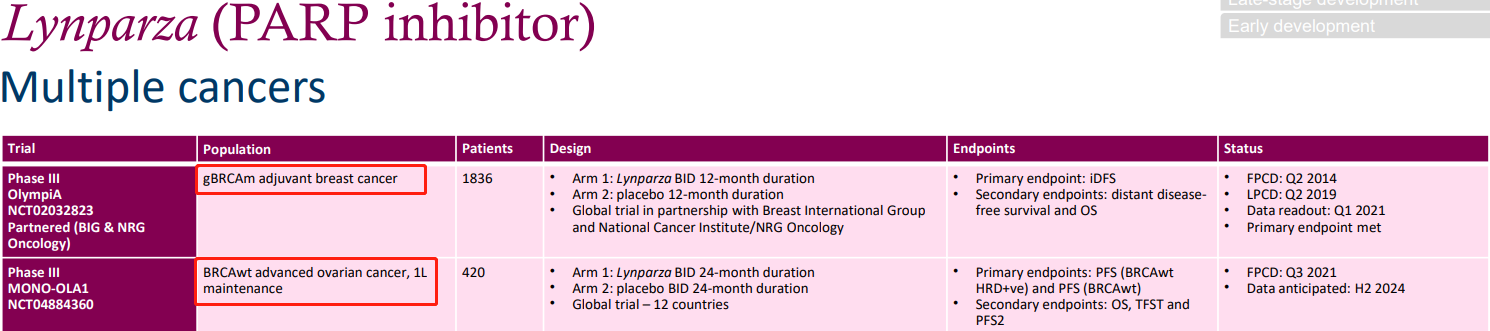
The above shows another Phase III clinical trial, NCT04884360, which is an international, multicenter, randomized, double-blind, placebo-controlled study to assess the safety and efficacy of maintenance olaparib compared to placebo in patients with BRCAwt who have Stage III or IV advanced high-grade serous or endometrioid ovarian cancer. These patients have achieved complete or partial remission after first-line platinum-based chemotherapy. The clinical trial is currently recruiting 420 patients, with the olaparib treatment group receiving a dosage of 300mg bid (n=280). The primary endpoints include: determining the advantage of olaparib as a maintenance treatment compared to placebo in terms of progression-free survival (PFS) in patients with Stage III/IV ovarian cancer with BRCAwt HRD-positive tumors after achieving complete remission/partial remission (CR/PR) from first-line platinum-based chemotherapy; and evaluating the same in patients with Stage III/IV ovarian cancer with BRCAwt tumors.
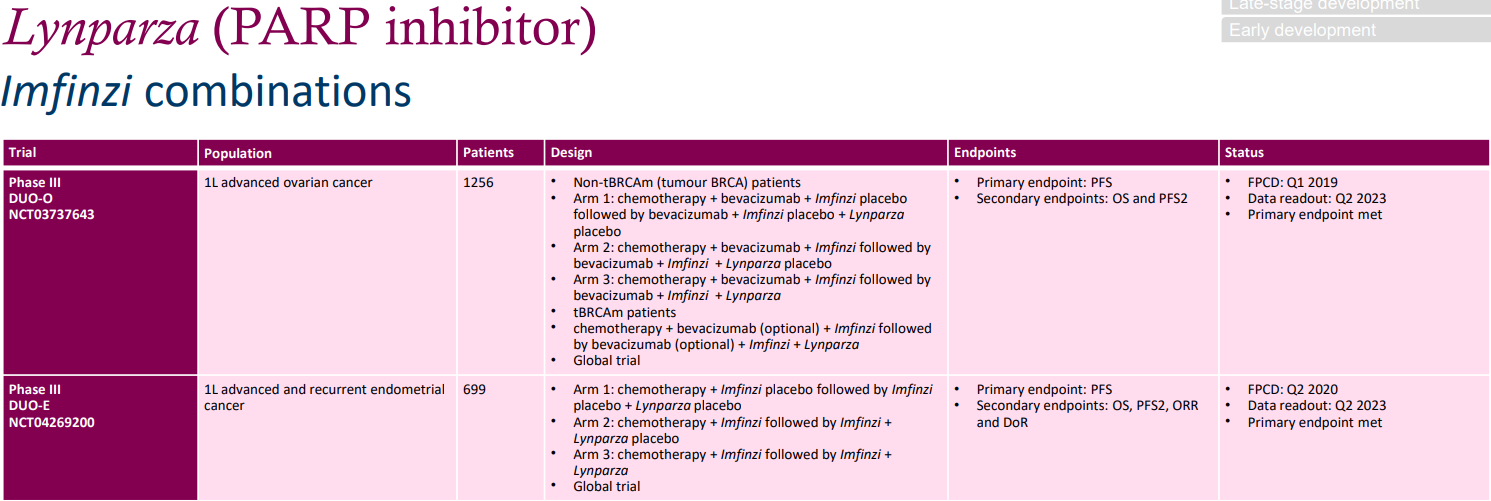
AstraZeneca's PD-L1 immunotherapy drug IMFINZI has been approved by the FDA for marketing, designed to treat patients with locally advanced non-small cell lung cancer (NSCLC) who are unable to undergo surgical resection. Multiple phase III clinical trials are currently underway.
Two phase III trials involving a combination with Olaparib serve as frontline treatments for patients with late-stage ovarian cancer and late-stage and recurrent endometrial cancer. They have respectively recruited 1256 and 699 patients. The primary endpoint is progression-free survival (PFS), with secondary endpoints including overall survival (OS) and PFS2, among others. Currently, both phase III trials have met their primary endpoints.
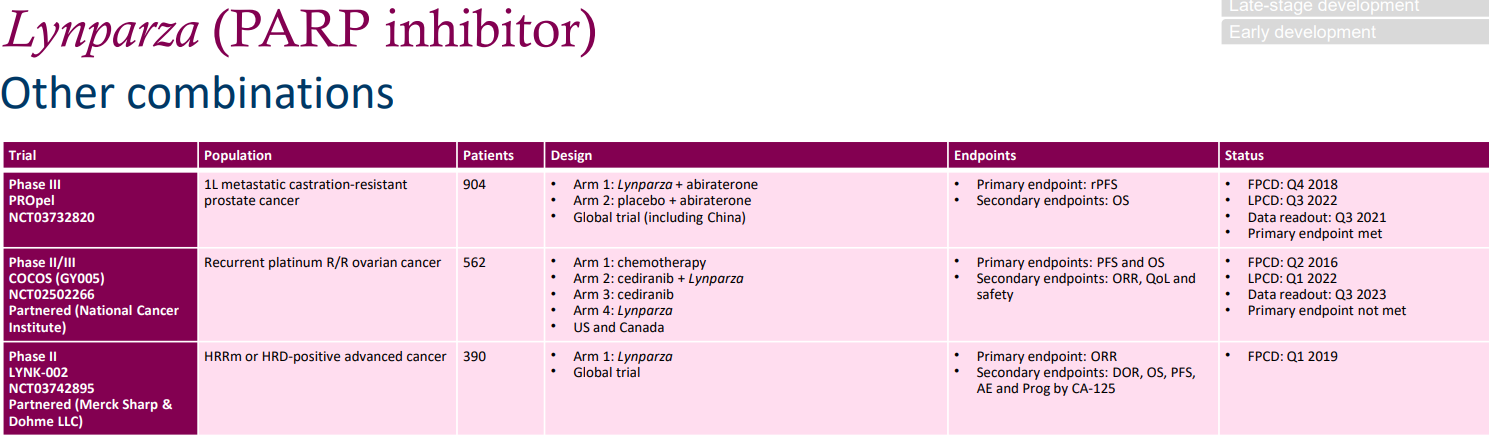
The three clinical trials in the graph below utilize a combination of Olaparib and other drugs. The phase III PROpel trial combines Olaparib with Abiraterone for the frontline treatment of metastatic castration-resistant prostate cancer (mCRPC). The doses are 300mg bid for Olaparib and 1000mg Qd for Abiraterone Acetate. The Q3 2021 data shows that the primary endpoint (rPFS) has been met. The phase II/III COCOS trial uses Olaparib in combination with a pan-VEGFR inhibitor to treat patients with recurrent platinum-resistant/refractory (R/R) ovarian cancer. The data shows that the primary endpoint (PFS, OS) has not yet been met.
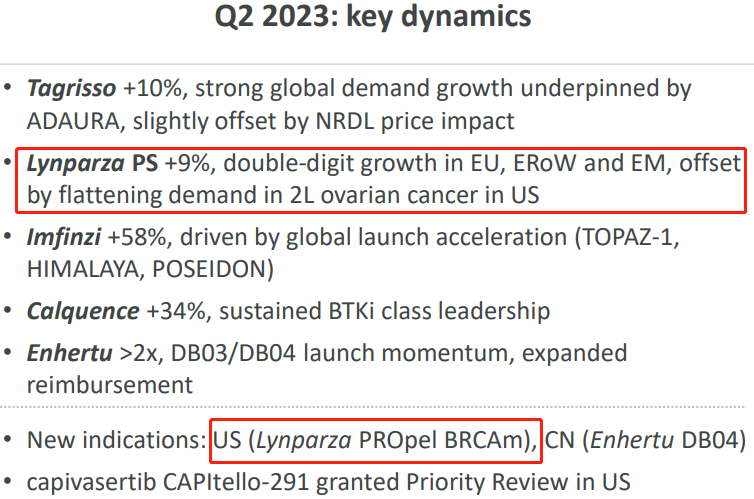
In summary, Lynparza PS +9%, achieving double-digit growth in Europe and other emerging markets, offsetting the impact of flatlining 2L ovarian cancer demand in the United States. Exploration of new indications mainly involves the development of mCRPC in the PROpel clinical trial. Androgen deprivation therapy is the foundational treatment for advanced prostate cancer, but after a median treatment duration of 18 to 24 months, nearly all patients progress to mCRPC. There is currently a lack of effective treatment for mCRPC and the prognosis is poor. Analysts estimate that this mCRPC indication alone could yield $30-50 billion in revenue for PARP inhibitors in the United States alone. In April of this year, the FDA convened a panel of experts to review the use of Lynparza in newly-diagnosed advanced prostate cancer patients. Lynparza is likely to become the first PARP inhibitor approved for first-line treatment of mCRPC patients in the United States.
Developing the Next Generation of PARP inhibitors: High Selectivity for PARP1 + Brain-Penetrating PARP Inhibitors
The first-generation of PARP inhibitors often have numerous deficiencies, including poor water solubility, low bioavailability, constrained tissue distribution, inability to cross the blood-brain barrier, and a risk of hematological toxicity, which to some extent limit their clinical applications. Therefore, there is a clinical need for the second generation of PARP inhibitors that have a higher selectivity for PARP1 and reduced adverse effects.
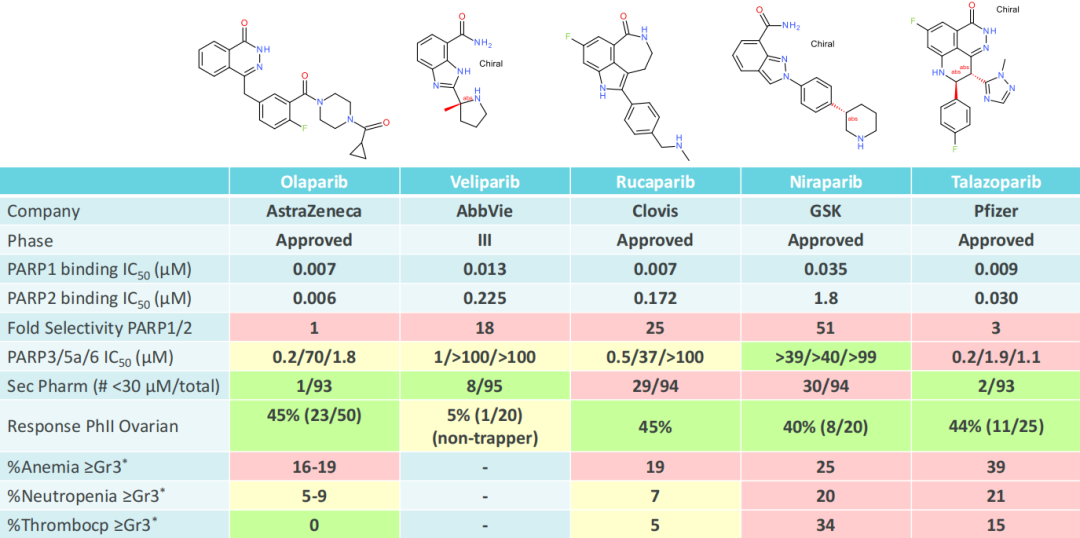
As shown in the figure above, the first-generation PARP inhibitors are dual inhibitors of PARP1 and PARP2. However, in the case of homologous recombination repair defects, only the participation of PARP1 is required. PARP2 is related to hematological effects, which are the main clinical adverse events observed in the first-generation PARPi. Among the four FDA-approved inhibitors, the selectivity of PAPR1/2 is less than 100 times, and all have ≥3 grade hematological adverse reactions, such as anemia, neutropenia, and thrombocytopenia. Studies in knockout mice also show that PARP2 is related to hematological toxicity, and the double knockout of PARP1/2 in embryos is lethal. Murai et al. and Ronson and others have shown that synthetic lethal BRCA mutations are entirely driven by PARP1, and the capture of PARP2 into DNA is not necessary for synthetic lethality.
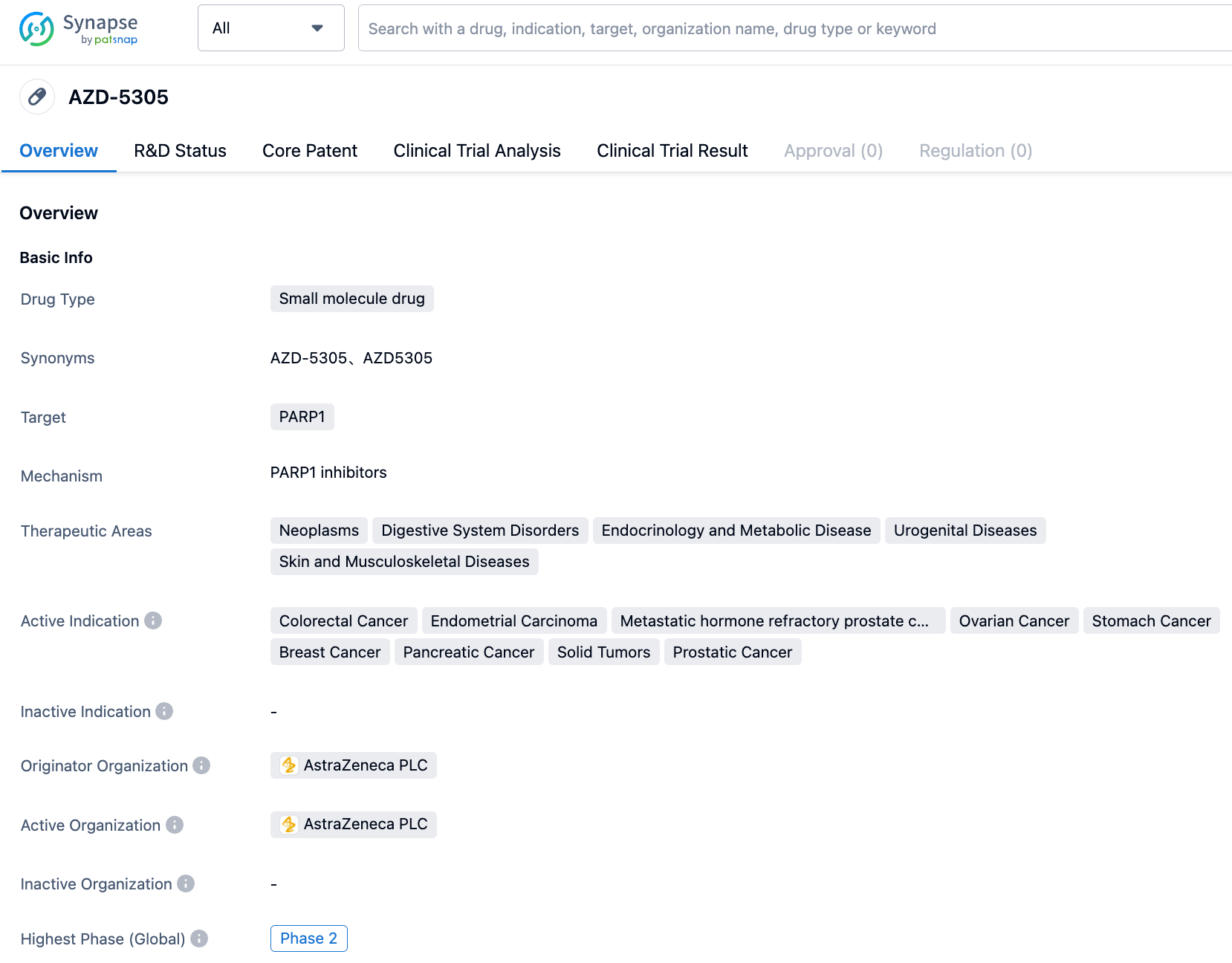
AstraZeneca first developed the highly selective PARP1 inhibitor AZD5305 (Phase I/II). Its current clinical indications include prostate cancer and solid tumors. The plasma clearance (CLp) of AZD5305 is very low in mice, rats, dogs, and cynomolgus monkeys, at 0.23, 1.1, 0.33, and 0.84 mL/min/kg respectively, and the steady-state distribution volumes (Vss) for mice, rats, dogs, and monkeys are 0.17, 0.38, 0.30, 0.38 L/kg. The oral bioavailability of AZD5305 is high in all species, consistent with its low hepatic clearance rate and high absorption. The low unbound clearance coupled with a high potency allows for a lower effective in vivo dosage.
However, the current clinical trial has not revealed the dosage of AZD5305. As of July 2023, only two clinical registry entries exist: NCT05938270 and NCT05797168. NCT05797168 comprises Phase I/IIa studies of AZD5335 as a monotherapy and in combination with anti-cancer drugs in patients with solid tumors. AZD5335 is a FRα ADC drug developed by AstraZeneca, with AZD5305 as an adjunctive agent. NCT05938270 also involves combination therapy with darolutamide.
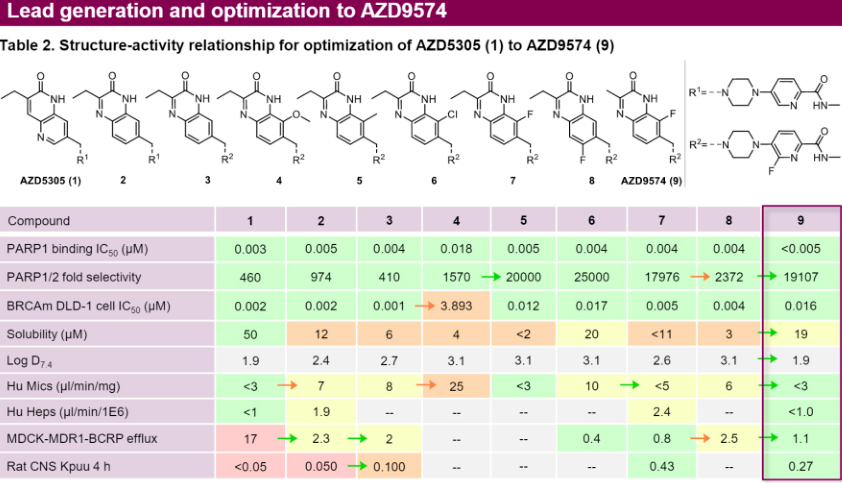
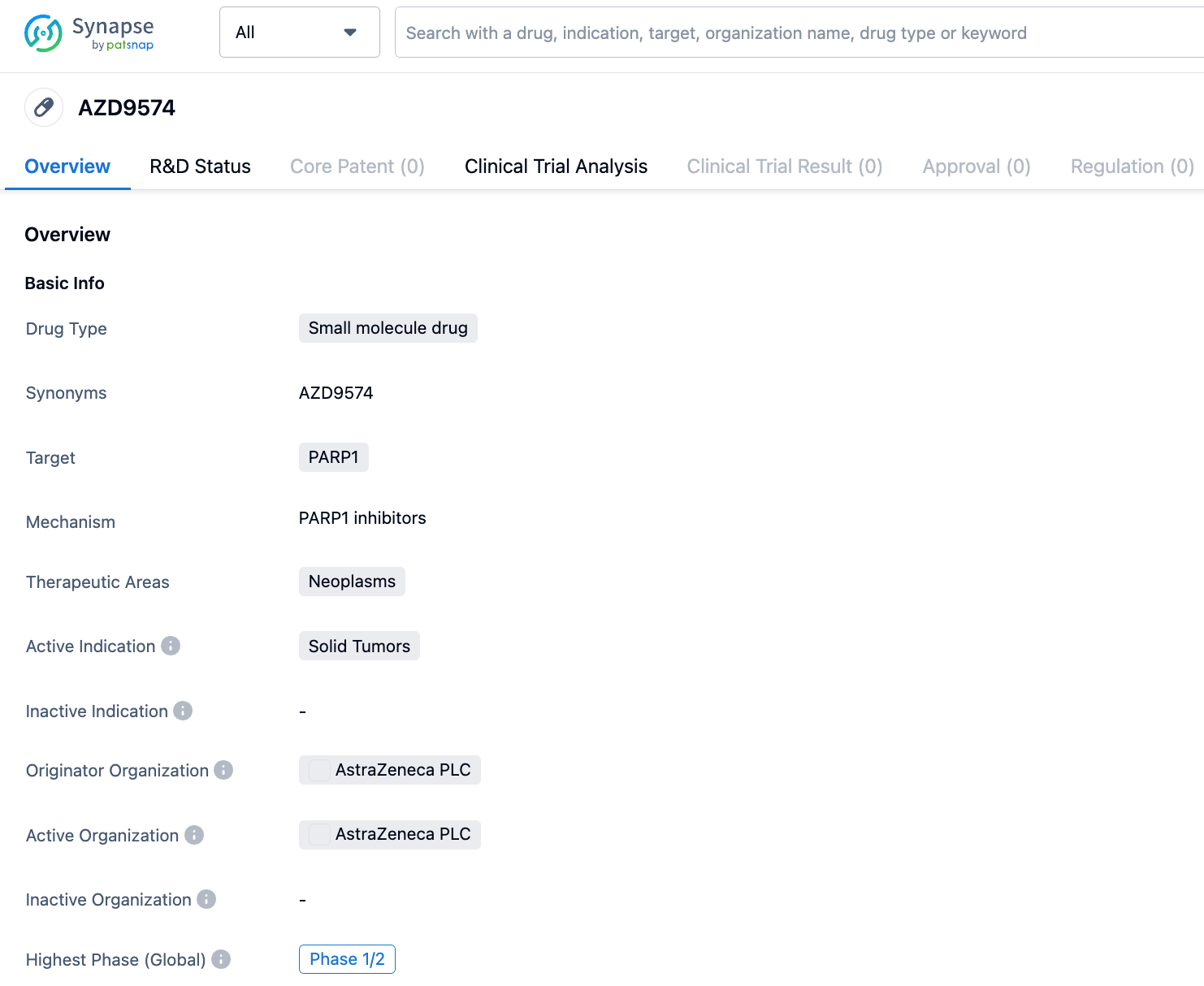
AstraZeneca has also developed the brain-penetrant PARP1 inhibitor AZD9574, which is over 8000 times more selective for PARP1 than it is for PARP2/3/5a/6. AZD9574 is a structural modification of AZD5305 against with targeted changes, significantly reducing efflux, with RatCNS Kpuu 4h improving from less than 0.05 to 0.27. Its limitation is that its solubility has decreased from 50μM to 19μM. However, despite the decrease in solubility, the IC50 is below 5nM, which is still enough to effective in the body.
AZD9574 is currently in Phase I/II clinical trials for solid tumors. A clinical study NCT05417594 has been registered: A study of AZD9574 as monotherapy and in combination with anticancer drugs (temozolomide) for the treatment of advanced malignant solid tumors.
AstraZeneca PARP inhibitor Intelligence Data
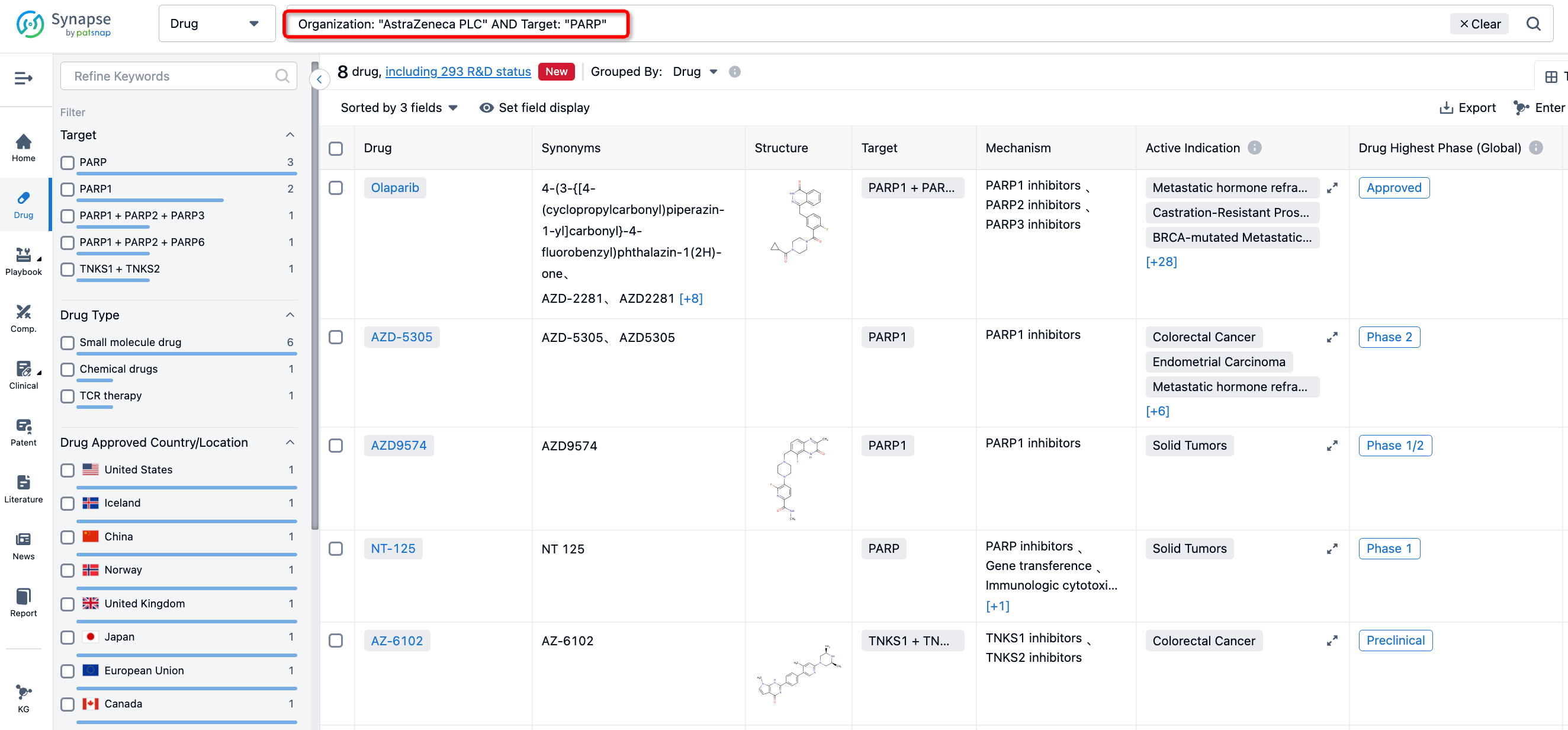
Search for PARP and AstraZeneca in the Synapse database to get a general idea of AstraZeneca's current focus on PARP based on its recent patent applications. For instance, AZ disclosed patent WO2023118085 on June 29, 2023, which primarily includes the introduction of methods for the treatment of brain tumors and neuroblastoma using compounds such as AZD9574. Patent WO2023089527 showcases the implementation of the antitumor effect of combined use of AZD5305 and TROP-2's ADC drug DS-1062. Patent WO2022074617 introduces the combination of PARP1 inhibitor AZD5305 and DS8201. Both DS-1062 and DS-8201 are ADC drugs developed by AstraZeneca/Daiichi Sankyo.
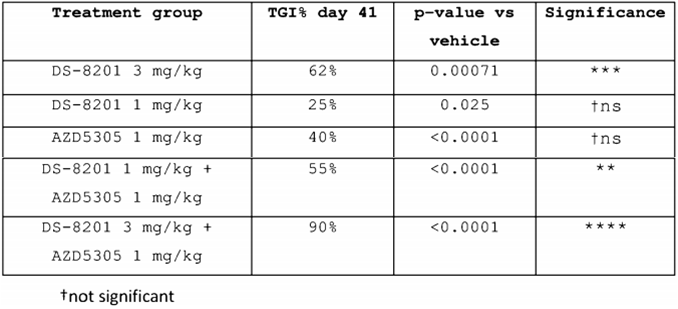
AstraZeneca compared the inhibitory effects of DS8201 and AZD5305 alone and in combination on tumors in patent CN116348115(A). In NCI-N87 xenografts, the TGI response (% on day 41) after treatment with DS8201 or AZD5305 alone or in combination is shown in the table below. In the fifth treatment group, when DS8201 at 3mpk was used in combination with AZD5305 at 1mpk, the % TGI on day 41 reached 90%, which was far better than the single drug group and the 1mpk DS8201 + 1mpk AZD5305 group, and the data was significant.
PARP inhibitor and Blood-Brain Barrier
The Blood-Brain Barrier (BBB) hinders the entry of drugs into brain tumors. Rucaparib and talazoparib are impacted by the efflux pumps of the BBB, limiting drug delivery. As such, rucaparib and talazoparib are ineffective for TMZ-sensitizing mouse brain tumor models. In contrast, niraparib penetrates the BBB and achieves sustained brain exposure. This favorable PK characteristic enables niraparib to achieve greater brain tumor growth inhibition compared to olaparib, which is a substrate of P-glycoprotein (ABCB1, MDR1). However, data suggests olaparib also permeates and is active in malignant brain tumors like glioblastoma multiforme (GBM). Therefore, whether a drug efflux transporter is a substrate cannot serve as a basis for predicting if PARPi can penetrate tumors in the brain. Studies on the PK and PD of individual drugs are both useful and necessary.
Summary
Based on the latest financial reports and clinical data from AstraZeneca, there are some key focus areas in their layout for PARP. PARP inhibitors had once brought about a "decade breakthrough" for ovarian cancer, and in search for new areas of business growth, AZ is primarily seeking the approval for the use of olaparib in metastatic prostate cancer. The main combination use of olaparib is in conjunction with PD-L1 antibody IMFINZI and abiraterone acetate.
They have also developed a new generation of PARP1 selective inhibitors, AZD5305 and a brain-penetrating highly selective PARP1 inhibitor AZD9574. However, the clinical dosage remain undisclosed and the direction of clinical trials has been more focused on combination therapy. Especially, signals disclosed in July this year and information from patents are enhancing the combination of highly selective PARP1 inhibitors with FRα ADCs, DS-1062, DS-8201 and other ADC drugs.
Whether the poor efficacy of second-generation PARP inhibitors is due to their single-drug therapy, or it is based on commercial factors, remains to be seen until more data is disclosed.