Can Arvinas lead the treatment of neurodegenerative diseases?
Since 2013, companies such as Arvinas, C4 Therapeutics, and Kymera Therapeutics have been established, focusing on the development of PROTAC technology. Pharmaceutical giants like Merck, Genentech, Pfizer, and Novartis have also entered this field. However, before 2020, several questions remained unanswered:
1.Is PROTAC safe and effective in the human body?
2.Will PROTAC cause off-target effects in the human body?
3.Does PROTAC retain the pharmacokinetic characteristics of small molecule drugs?
4.Can PROTAC's main feature of being effective against drug resistance be validated in large-scale Phase III clinical trials?
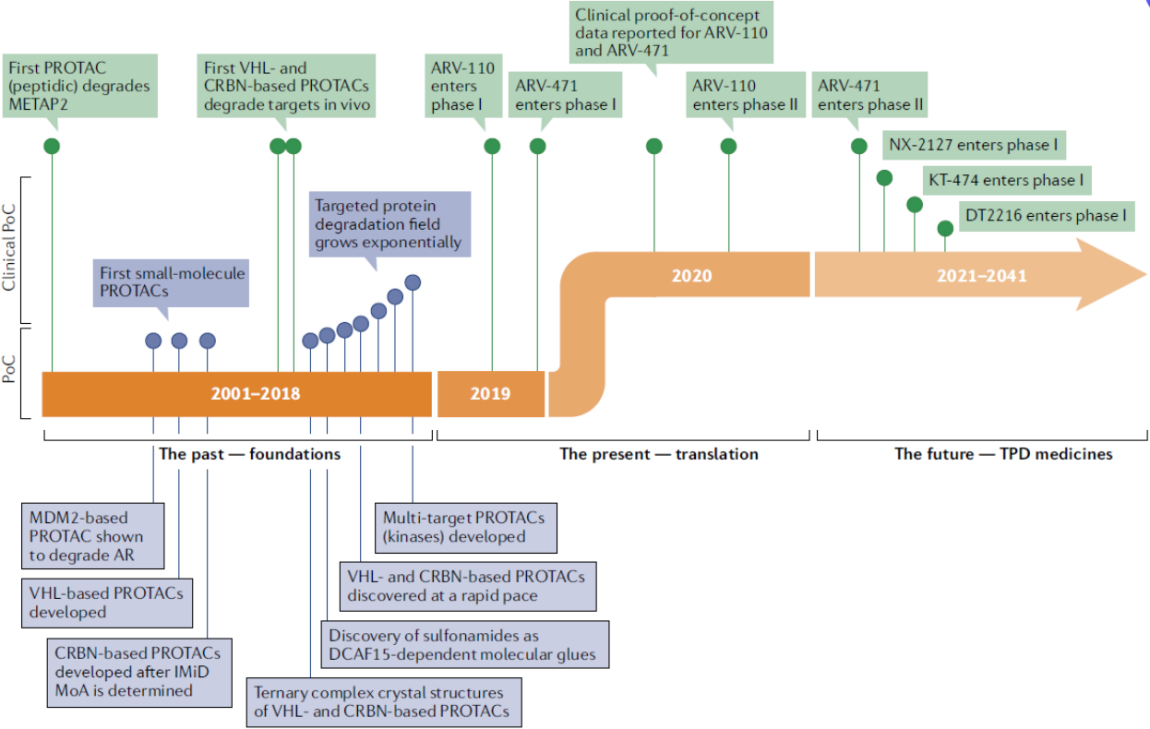
In 2019, Arvinas Inc. first advanced ARV-110 and ARV-471 into phase 1 clinical trials, and the clinical data released in 2020 provided clear and affirmative answers to the aforementioned three questions.
From then on, the wheels of fate began to turn. In July 2021, Pfizer and Arvinas Inc. entered into a global partnership, reaching a total amount of $2.4 billion. In June 2019, Nurix and Gilead Sciences initiated a global strategic partnership worth $2.345 billion to develop innovative anti-cancer drugs capable of degrading pathogenic proteins using Nurix's PROTAC technology platform. In January 2020, Nurix and Sanofi embarked on a strategic partnership worth $2.5 billion to discover, develop, and commercialize a series of innovative targeted protein degradation drugs for patients with challenging diseases.
Pioneer Arvinas has successfully advanced ARV-471 project to Phase III clinical trials, ARV-110 is in Phase II for the treatment of prostate cancer, and ARV766 is in Phase I/II; this has injected a strong stimulus into the entire PROTAC field, soaring the enthusiasm for clinical development to new heights.
Although the first batch of PROTAC pipelines that entered the clinical stage mostly chose cancer as the indication, theoretically PROTAC is capable of degrading any target, and its potential is far beyond this. Therefore, amidst the many PROTAC pipelines targeted at cancer, two molecules stand out-- because they have chosen indications other than cancer: Nurix Theraputics' BTK degrader NX-5948 (pre-clinical development for rheumatoid arthritis), and Kymera Theraputics' IRAK4 degrader KT-474, used to treat various immune inflammations such as rheumatoid arthritis. Arvinas has chosen neurodegenerative diseases.
In the United States, the death toll from neurodegenerative diseases is devastating, and the situation is becoming increasingly severe with the aging of the population. According to estimates from the Alzheimer's Association, 6.7 million people over the age of 65 in the US have Alzheimer's disease, the most common form of dementia. This number could potentially double by 2050. The incidence of other neurodegenerative diseases such as Parkinson's disease and Huntington's chorea is also on the rise. The following figure presents several common types of neurodegenerative diseases.

CNS targets have long been considered "incurable." Researchers have been working to design small molecule therapeutics that can cross the blood-brain barrier and reach intracranial targets. Gene-based treatments and monoclonal antibodies cannot cross the blood-brain barrier, hence the small molecule PROTAC® degraders could provide important therapeutic opportunities for potential treatments of neurodegenerative diseases.
PROTAC® protein degraders utilize a new mechanism to target proteins associated with neuropathology and functional impairments. Unlike typical small molecules, they don't just block target proteins. Instead, they utilize a natural intracellular pathway known as the ubiquitin-proteasome system to target pathogenic proteins.
Importantly, some PROTAC® protein degraders are able to cross the blood-brain barrier and can be designed to only target pathogenic proteins without affecting healthy proteins. Once inside the brain, these drugs are able to degrade the pathogenic proteins.
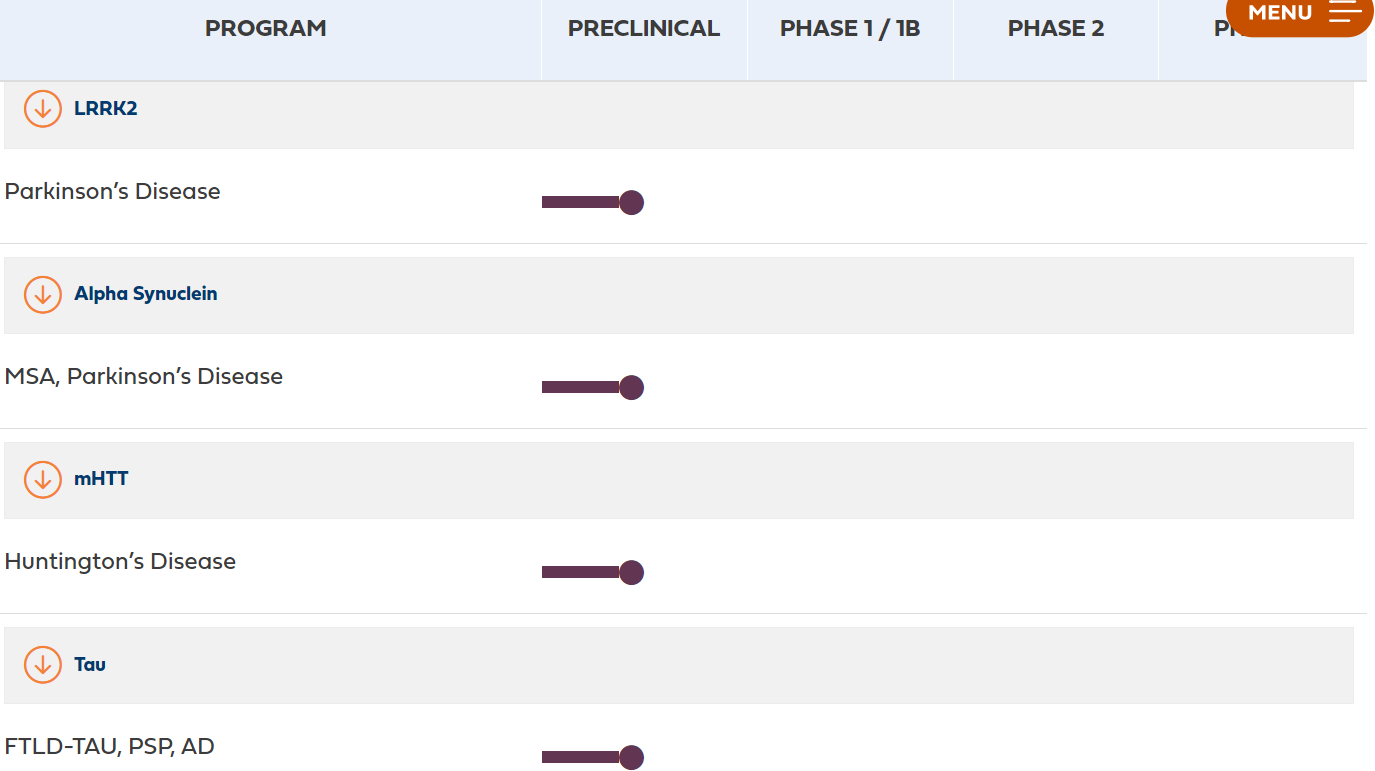
The neurodegenerative disease pipeline of ARVINAS is in the preclinical stage
Arvinas is currently conducting preclinical studies on protein degradation for neurodegenerative diseases. The graph below shows several target proteins published on the company's official website, including LRRK2 protein and α-synuclein for Parkinson's syndrome, mutant HTT protein for Huntington's disease, and pathogenic tau protein for Alzheimer's disease. The LRRK2 project is expected to complete IND (Investigational New Drug) studies this year.
Parkinson
Mutations in the LRRK2 (Leucine-Rich Repeat Kinase 2) gene are one of the most common genetic risk factors for Parkinson's disease, associated with both familial (hereditary) and sporadic (no family history) cases. Certain LRRK2 mutations are associated with increased LRRK2 kinase activity and expression, as reported in some Parkinson's patients and preclinical studies, further indicating potential roles in the onset and progression of the disease.
Human genetic and preclinical animal model data suggest that reducing LRRK2 protein by 50% might impact the pathology and functional deficits of Parkinson’s disease. Therefore, reducing LRRK2 in the brain could potentially be beneficial for treating this neurodegenerative disorder.
Inputting the keywords: LRRK2 and ARVINAS in the Patsnap patent database, three WO patents were found-- namely WO2021194878, WO2021194879, and WO2022198112. All three patents use CRBN as the E3 ubiquitin ligase ligand; initial linkers include ethylene glycol chains and other long chains, but later introduced some rigid tetrahedral ring fragments to boost oral bioavailability. In addition, patent WO2020041331 was found using targeted α-synuclein degradation.
As shown in the figure below, compound 12 in patent WO2021194879 can induce degradation (DC50 < 10 nM, Dmax = 70% or higher) of HiBit-tagged wild-type LRRK2 and G2019S mutant in HEK-293 cells.
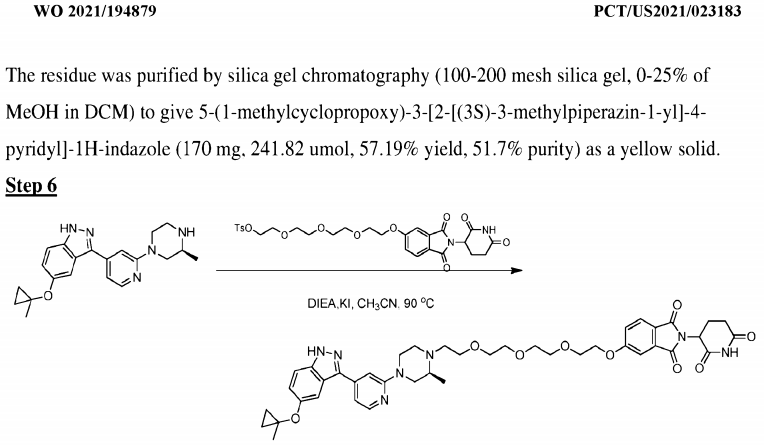
The compound in Example 22 of Patent WO2021194878 can induce degradation of wild-type LRRK2 and LRRK2 Gly2019Ser mutants tagged at the C-terminus with HiBit in HEK-293 cells (DC50 <10 nM, Dmax = 70% or higher).
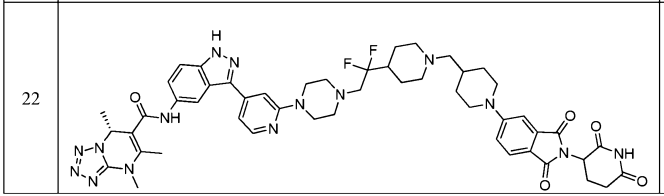
The compound 61 in the latest updated patent WO2022198112 can induce the degradation of HiBit tagged LRRK2 wild type and -G2019S mutant (DC50 < 10 nM, Dmax = 70% or higher) in HEK-293 cells.
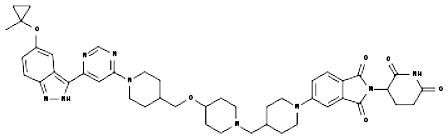
Alzheimer disease
Many studies on the causes of Alzheimer's disease focus on tau protein, which is a protein found in axons. Axons are components of neurons that send signals to other cells in the form of electrochemical waves, which are required for healthy brain function. Mutated or pathogenic tau protein detaches from microtubules, which typically stabilize axons, and negatively impacts neurons and supportive neuroglia by forming pathological tau protein. This eventually leads to cellular dysfunction and death, and it also forms "neurofibrillary tangles," which are a hallmark pathological characteristic of Alzheimer's disease.
In preclinical studies, Arvinas has demonstrated that the form of tau associated with neuronal tangles can be targeted with PROTAC® protein degradation drugs. In the murine model of Cowdria, PROTAC® degraders degraded 95% of the pathological tau. Beyond Alzheimer's disease, other neurodegenerative diseases characterized by tau accumulation, or tauopathies, may also be candidates for PROTAC protein degrader drugs, such as Frontotemporal dementia and Progressive Supranuclear Palsy.
In the patsnap patent database, searches for keywords Tau and ARVINAS found patents WO2018102067, WO2021011913, US2023086457.
The compound in Embodiment 4 of patent WO2018102067 degrades tau protein in human neuroblastoma SK-N-SH cells (11-300 nM). In a pharmacodynamic study, male B16 wild-type mice (bilateral intrahippocampal injection dose of 1mg /ml) reduced tau protein in the hippocampal brain.
Compound 382 in patent WO2021011913 degrades Tau induced by doxycycline in T-Rex CHOK1-Tau P301L cells (1 mcM for 48 h > 50%). After Wes Capillary Gel Electrophoresis measurement, the compound can induce the degradation of pathological Tau in the brains of tauopathic mice (over 95% when intravenously dripped at 30 mg/kg), and can dose-dependently reduce the level of pathological Tau in the hippocampal brain of mice (0.3, 1, 3 and 15 mg/kg intravenous drip after 24 hours).
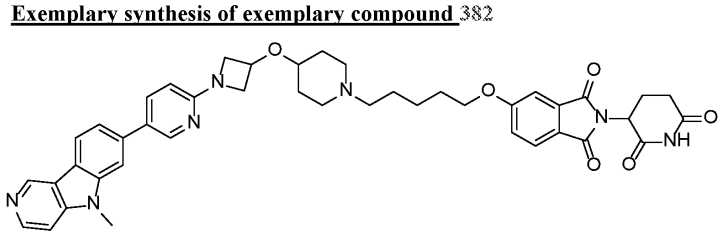
Huntington Disease
Huntington disease is a rare but devastating illness associated with mutations in the HTT (huntingtin protein) gene. This mutation results in an altered version of a protein referred to as mHTT, leading to protein aggregation, abnormal cleavage, and ultimately, the loss of striatal neurons located in motor-control related areas of the brain.
Arvinas has developed PROTAC® molecules that can target and degrade mHTT in cells (including neurons) while preserving healthy HTT. In ongoing preclinical studies with a Huntington's disease mouse model, Arvinas is investigating the role of clearing pathological mHTT protein.
Summary
Judging from the patents currently made public by ARVINAS, the exploration of the treatment for Parkinson's disease is in the fastest stage, involving two targets, and four WO patents have been made public, with the DC50 of the key compound being less than 10 nM, and Dmax being 70% or higher. On its official website, ARVINAS mentioned that the first CNS-penetrating PROTAC® submitted for IND/CTA application will appear before the end of 2023. We look forward to the expansion of PROTAC technology in new indications, bringing good news to patients.
Reference
1.https://www.arvinas.com/research-and-development/pipeline/.
2.https://endpts.com/sp/protac-protein-degraders-how-arvinas-is-pioneering-potential-next-generation-treatments-for-neurodegenerative-diseases/.