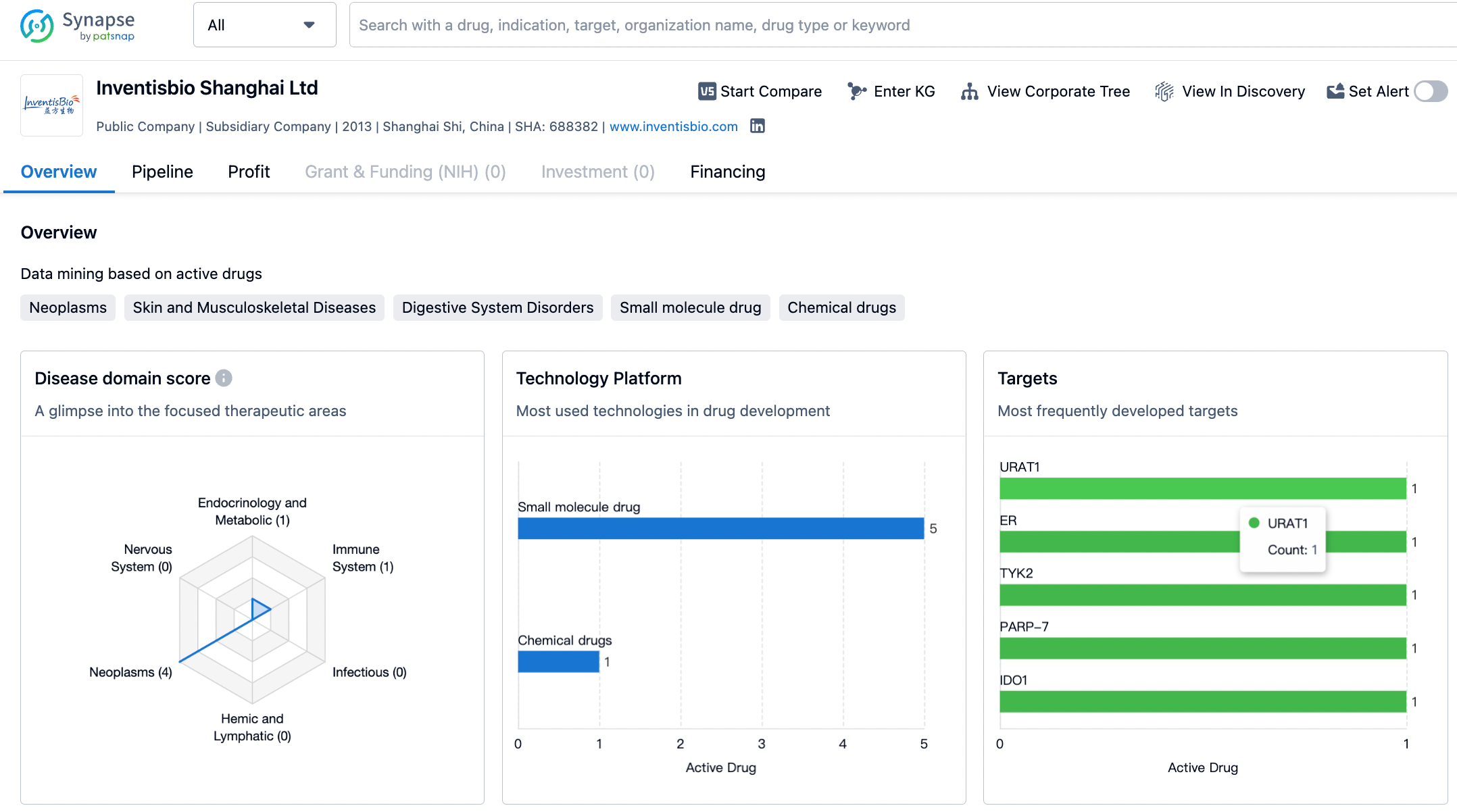
Sales fall short of expectations, how can KRAS G12C be differentiated in R&D?
On August 3, 2023, Inventisbio Shanghai Ltd. announced a Licensing and Collaboration Agreement with Chia Tai Tianqing Pharmaceutical Group Co., Ltd. for the KRAS G12C inhibitor D-1553 (Garsorasib). According to the agreement, the company grants Chia Tai Tianqing exclusive licensing rights to develop, register, produce, and commercialize D-1553 in mainland China during the term of the agreement. Chia Tai Tianqing will pay Inventisbio up to 550 million yuan in upfront and milestone payments, and will pay Inventisbio a royalty on net annual sales.
On the same day, Amgen also released its half-year financial report for 2023. LUMAKRAS only had a semi-annual sales amount of $151 million, with quarterly sales decreasing by 5% year-on-year while the volume increased by 12%. The report pointed out: the sales revenue remained the same year-on-year, and the 20% volume increase was offset by lower net selling prices and inventory levels. The main reason for the less-than-expected volume is the current scarcity of patients, with only 7000 patients in the U.S. and 20000 outside the U.S. In addition to the combination of KRAS G12Ci and PD1, the development of new indications such as colorectal cancer and other solid tumors is crucial.
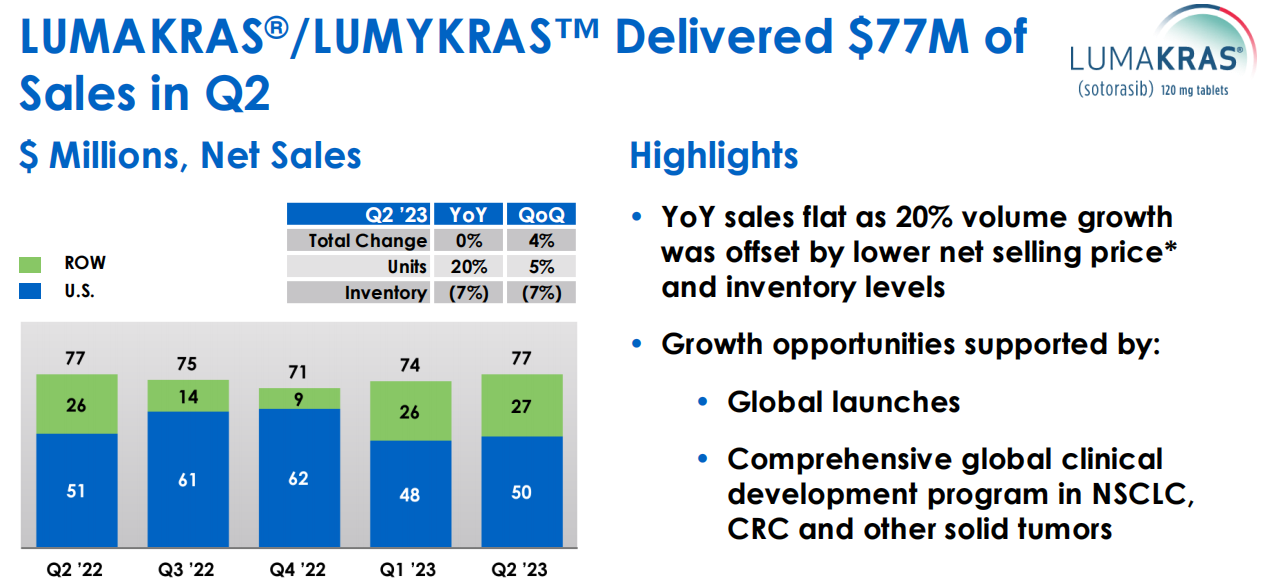
LUMAKRAS Sales Data in Amgen's Semi-annual Financial Report for 2023
With KRAS, a previously considered untouchable target, being cracked open, the KRAS G12C inhibitors of Amgen and Mirati are now on the market. The question is, how can we further optimize treatment outcomes for patients? One key consideration is the brain penetrance of these inhibitors, as treating and preventing brain metastasis is crucial for patient prognosis. The development of brain metastasis in lung cancer is typically correlated with shorter survival time. In addition, Gusamrin and his colleagues reported a significant increase in the symptoms of fatigue, headaches, and depression after diagnosing brain metastasis, indicating the importance of central nervous system disease control in maintaining patient's quality of life.
Of the two drugs that are already on the market, the clinical trial of Sotorasib excluded patients with active CNS metastasis; the paper - "Activity of MRTX849 in Brain Metastatic Tumors", analyzed preclinical models and clinical data of patients with KRAS G12C mutational non-small cell lung cancer. Sabari and his colleagues first conducted a retrospective analysis of brain metastasis in patients with KRAS G12C mutational non-small cell lung cancer, then a comprehensive overview of the pharmacology and preclinical data of Adagrasib, followed by a case study of successful response to Adagrasib in two previously untreated patients with brain metastasis.
Among all patients with KRAS G12C mutation in the queue, 40% (n=150) demonstrated brain metastasis, indicating high propensity of these patients towards tumor progression in the CNS system. There are some limitations as well, for instance, comparisons were only made within KRAS mutated NSCLC, without continuous CNS imaging for more definitive evidence. Nevertheless, 40% is a convincing percentage that aligns with previous research.
Sabari and colleagues conducted comprehensive pharmacological and preclinical studies of Adagrasib, emphasizing its good PK properties, including penetration into the CNS system. In mouse models, the IC50 of Adagrasib for inhibiting P-glycoprotein was 980 nmol/L, suggesting that it could potentially bypass the limitations of the blood-brain barrier by inhibiting its own excretion at physiological concentrations. Eight hours after oral administration of 200 mpk Adagrasib, the total plasma concentration, free plasma concentration, and cerebrospinal fluid (CSF) concentration in mice were 8600, 43, and 52 nmol/L, respectively. At a dose level of 200 mg/kg in mice (which is equivalent to the average steady state in humans at a Phase II dose of 600 mg, twice daily), the ratio of unbound drug concentration in brain and unbound plasma (Kp,uu) was about 1 at 8 hours, indicating significant penetration into the CNS system at this dosage.
Lastly, the authors presented two case studies of previously untreated brain metastasis patients who were orally administered Adagrasib as part of the KRYSTAL-1 study. Consistent with preclinical data, in these two untreated brain metastasis patients, Adagrasib CSF concentrations exceeding cellular IC50 of the drug were observed in continuous imaging studies, and both patients showed corresponding remissions in brain lesions. The average Kp,uu in these two patients was 0.47, which is consistent with observations from preclinical models and exceeds the value of other known tyrosine kinase inhibitors with CNS system activity.
Journalist Conor Killmurray's Personal Page
This brain metastasis research may face criticism as it only involved two study patients. However, the efforts to extend the study drugs to patients with brain metastases should be applauded, as it produces invaluable data. Although the Phase Ib portion of the trial initially only permitted patients with stable, asymptomatic, untreated (non-cerebellar/non-leptomeningeal) brain lesions smaller than 2 cm, it later expanded the criteria, removing the size restriction and brain lesions, and allowed up to 25 patients. Given that 40% of KRAS G12C mutant patients could experience brain metastases at any time, this number cannot be overlooked. While previous research traditionally hesitated to allow patients with brain metastases into studies, the Friends of Cancer Research Brain Metastases Working Group of the American Society of Clinical Oncology has urged for an improvement in inclusion for these patients, hoping to see a greater integration of patients with brain metastases, so that the trial patient population can represent the "real world" patient population.
Looking back at the Inventisbio deal, it is highly likely that Chia Tai Tianqing saw the potential of D-1553 for the treatment of intracranial (IC) disease and brain metastatic cancer patients.
On July 29, a commentator who focuses on the oncology field commented on the clinical performance of Inventisbio's D-1553 and concluded: The initial results of Phase I clinical trials indicate that garsorasib (D-1553) does not yet have a therapeutic or toxicity advantage compared to other KRAS G12C inhibitors.
Upon careful analysis of the clinical data, an effectiveness evaluation was conducted on 74 eligible patients. Among them, 6 patients also had intracranial (IC) disease, with stable and assessable brain metastases at baseline. The total IC efficacy rate of these patients was 17%, and the IC DCR was 100%; compared to the Phase 1/2 KRYSTAL-1 trial (NCT03785249), which studied the efficacy of adagarsib on non-small cell lung cancer patients, the IC ORR of the brain metastasis subgroup was 33%, and the DCR was 85%.
However, the KRYSTAL-1 trial subgroup studied 33 patients, and D-1553 only had 6 patients; the difference in the number of patients is particularly important. More clinical data from brain metastatic cancer patients and even head-to-head comparisons are still needed to demonstrate the difference or superiority between D-1553 and adagarsib.
The search in the Patsnap patent database using KRAS and InventisBio as keywords, shows that the structure of D-1553 is already public, and it is easy to find that this clinical molecule originates from patent WO2020233592 (A1). The earliest priority date for patent WO2020233592 is May 21, 2019, the international application date is May 20, 2020, and the international publication date is November 26, 2020. The domestic related patent CN112585129 (A) was granted on March 1, 2022, after 11 months of examination, and it is expected to expire on May 20, 2040. CN112585129 (B) includes activity test data for 186 compounds, and Compound 44 in Implementation Example 16 is precisely D-1553.
AZ mentions that early screening of brain-penetrant drugs generally requires a molecular weight (MW) < 450, a polar surface area (PSA) < 90 Å2, and LogD between 1-4. It has also been summarized that a more stringent PSA < 70 Å2 is needed. Further enhancement of brain penetration requires PSA < 90 Å2, hydrogen bond donors (HBD) < 3, LogD in the range of 2 ~ 5, and MW <500. The current guidelines for CNS drug design include a PSA < 76 Å2 (preferably within the range of 25 - 60 Å2) and HBD < 3 (ideally 0 or 1). Sotorasib (MW = 561, PSA = 92 Å2, HBD = 1, cLogp = 2.3), Adagrasib (MW = 604, PSA = 71 Å2, HBD = 0, cLogp = 3.6), and D-1553 (MW = 599, PSA = 119 Å2, HBD = 2, cLogp = 4.9) are all far away from these ideal characteristics. Inventisbio mentioned in the article that the lipophilicity (logD) of D-1553 is much lower than other KRAS G12C inhibitors. This reduced lipophilicity can increase the concentration of free drug in the plasma, ultimately aiding brain permeation. The consideration of brain-penetrating indications may also be due to the fact that the blood-brain barrier in cancer patients with brain metastases may be damaged compared to normal individuals, increasing the possibility of drug entry into the brain. The structure of D-1553 also evokes propofol, an anesthetic drug which needs to enter the brain to function.
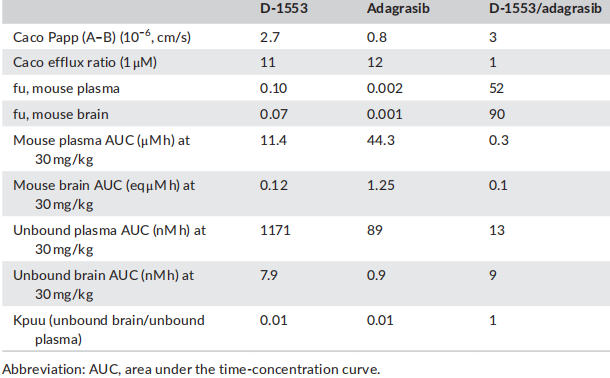
The effects of D-1553 and Adagrasib on the in vitro unbound brain exposure and brain Kpuu in mice.
The above figure is the data for mice in a paper published by InventisBio this year. Both D-1553 and Adagrasib are high efflux substrates. For efflux substrates, brain Kpuu is a more reliable parameter than CSF Kpuu to predict brain penetration efficiency, while CSF Kpuu tends to significantly overestimate the real brain penetration. Therefore, brain Kpuu is used to calculate the exposure of non-binding drugs to the central nervous system. Although there are significant differences in the free and total exposure in plasma and brain tissue of D-1553 and Adagrasib in nude mice at the same dose of 30 mg/kg, their brain Kpuu is also very close.
Adagrasib has already obtained POC in preclinical animal models for the treatment of brain metastases, and preliminary clinical data also support its follow-up research as a CNS penetration inhibitor. There are more details to explore. For example, the duration of the brain's response is still a question and we need to wait for the clinical results of the 25 KRYSTAL-1 brain metastasis patients. Moreover, although it is very bold to allow untreated brain metastasis patients to participate in the study, it must be admitted that many brain metastasis patients have undergone radiotherapy or surgical treatment when symptoms appear, and the damage of radiotherapy to the blood-brain barrier needs to be considered. What role can Adagrasib play in these specific treatment scenarios? Finally, there may be molecular heterogeneity between the primary and metastatic sites of the tumor, especially in the cerebral region. The difference in the tumor microenvironment has been reported, but how do we interpret this?
The patient is fortunate, as the FDA has already approved two KRAS G12C inhibitors, and there are a large number of KRAS G12C inhibitors in clinical stages both domestically and abroad. KRAS G12C, once considered "un-druggable," has now become one of the most popular targets for "druggable" drugs. The key next step to further improve patient prognosis will be assessing the brain penetration potential of these inhibitors, as treatment and prevention of brain metastasis are critical to patient prognosis. KRYSTAL-1 allows patients with untreated brain metastases to enroll, without considering the selection of easily treatable patients to make more impressive clinical data, which should be commended. Meanwhile, the two marketed drugs are both third-generation tyrosine kinase inhibitors (TKIs), and prospective interventional studies with continuous central nervous system imaging are the gold standard for assessing the central nervous system activity of TKI inhibitors.
Reference
1.Asfar S. Azmi et.al; Penetrating the central nervous system sanctuary of KRAS, a target once thought “undruggable”. Transl Lung Cancer Res 2023;12(4):665-668.
2.Yaolin Wang et al;D-1553: A novel KRASG12C inhibitor with potent and selective cellular and in vivo antitumor activity.Cancer Science. 2023;114:2951–2960.
3.CN112585129B.
4.https://www.targetedonc.com/view/garsorasib-results-add-to-the-growing-group-of-krasg12c-inhibitors-in-nsclc.