How to find the sequence of golodirsen?
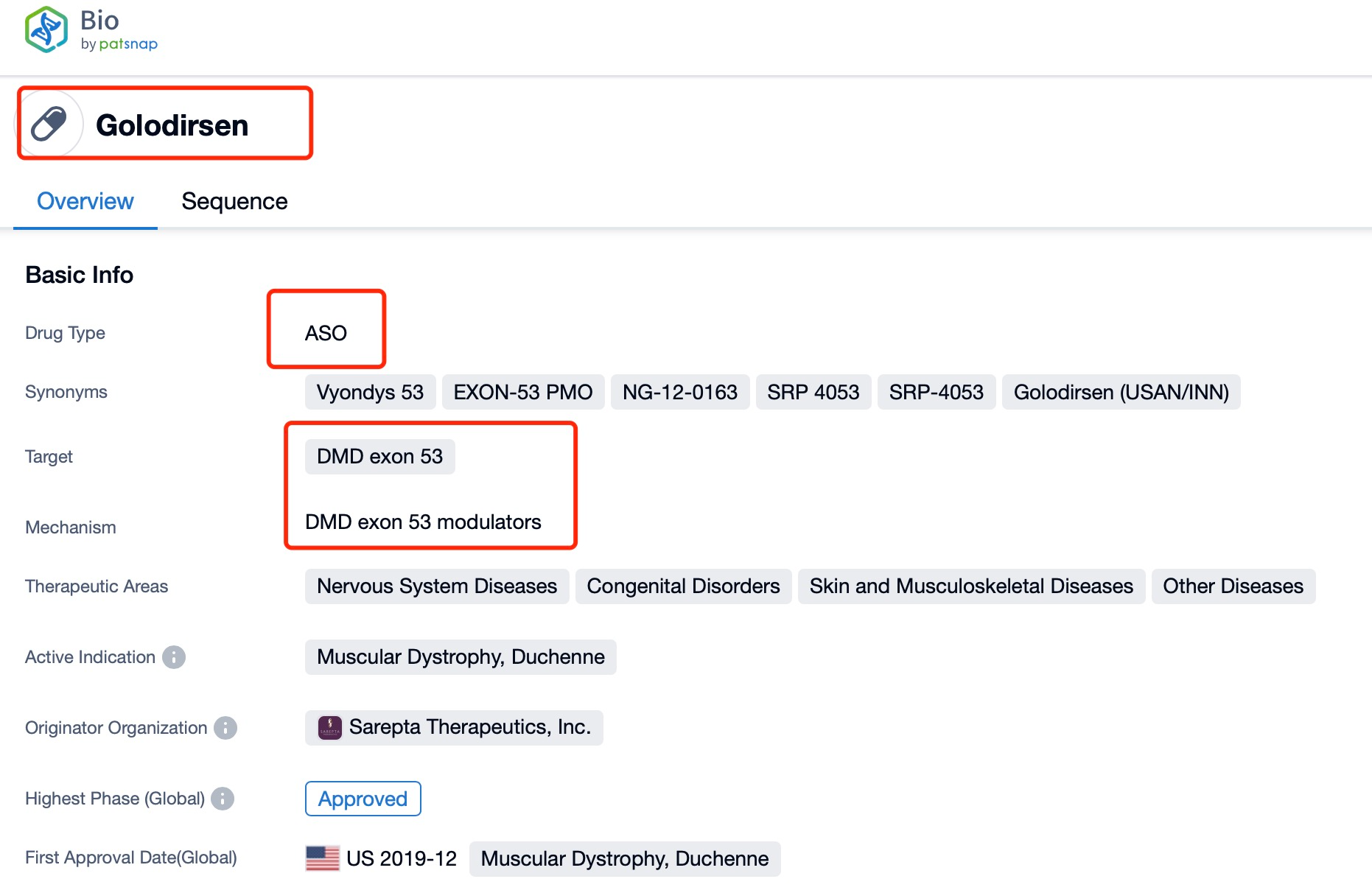
Golodirsen (Vyondys 53), developed by Sarepta Therapeutics, is a groundbreaking antisense oligonucleotide that represents a significant advancement in the treatment of Duchenne muscular dystrophy (DMD). This innovative therapy is designed to target and induce exon 53 skipping in the mRNA of the DMD gene, which is affected in DMD patients. By restoring the reading frame of the DMD gene, golodirsen enables the production of a functional, albeit truncated, dystrophin protein, which is crucial for muscle function and health.
DMD is a rare and severe genetic disorder characterized by progressive muscle weakness and degeneration. It is caused by mutations in the dystrophin gene that result in the absence or severe reduction of functional dystrophin protein. Golodirsen's targeted approach addresses the genetic root cause of DMD by using a phosphorodiamidate morpholino oligomer (PMO) that specifically binds to the pre-mRNA of the dystrophin gene, leading to the exclusion of exon 53 during mRNA splicing. This process results in the production of a shorter but functional dystrophin protein, which can mitigate the symptoms of DMD in patients with mutations amenable to exon 53 skipping.
Summary of Research Progress of Golodirsen
Golodirsen has undergone extensive clinical research, with a pivotal phase I/II trial demonstrating its ability to significantly increase dystrophin levels in patients with DMD amenable to exon 53 skipping. This trial included both a randomized, double-blind, placebo-controlled portion, and an open-label extension, showing promising results in terms of safety and biological activity. Based on these positive outcomes, golodirsen received accelerated approval from the FDA in December 2019, marking a significant milestone in the treatment of DMD. The drug is currently in phase III clinical development globally, further evaluating its efficacy and safety in a broader patient population.
The global market for DMD treatments is evolving, with golodirsen being one of several exon-skipping therapies in development. Other drugs targeting the same mechanism include eteplirsen (Exondys 51) and viltolarsen (Viltepso), both of which also aim to induce exon skipping but target different exons (exon 51 and exon 53, respectively). The competition in this space is focused on addressing the specific mutations that can be corrected by each therapy, with golodirsen targeting approximately 8% of DMD patients with a mutation amenable to exon 53 skipping.
Sequence Characteristics of Golodirsen
Golodirsen is a phosphorodiamidate morpholino oligomer (PMO), a class of synthetic oligonucleotides designed for high stability and resistance to enzymatic degradation. The sequence of golodirsen is meticulously crafted to bind to the pre-messenger RNA (pre-mRNA) of the dystrophin gene (DMD) at a precise location corresponding to exon 53. This binding facilitates the skipping of exon 53 during the splicing process, which can result in the production of a shorter but functional dystrophin protein.
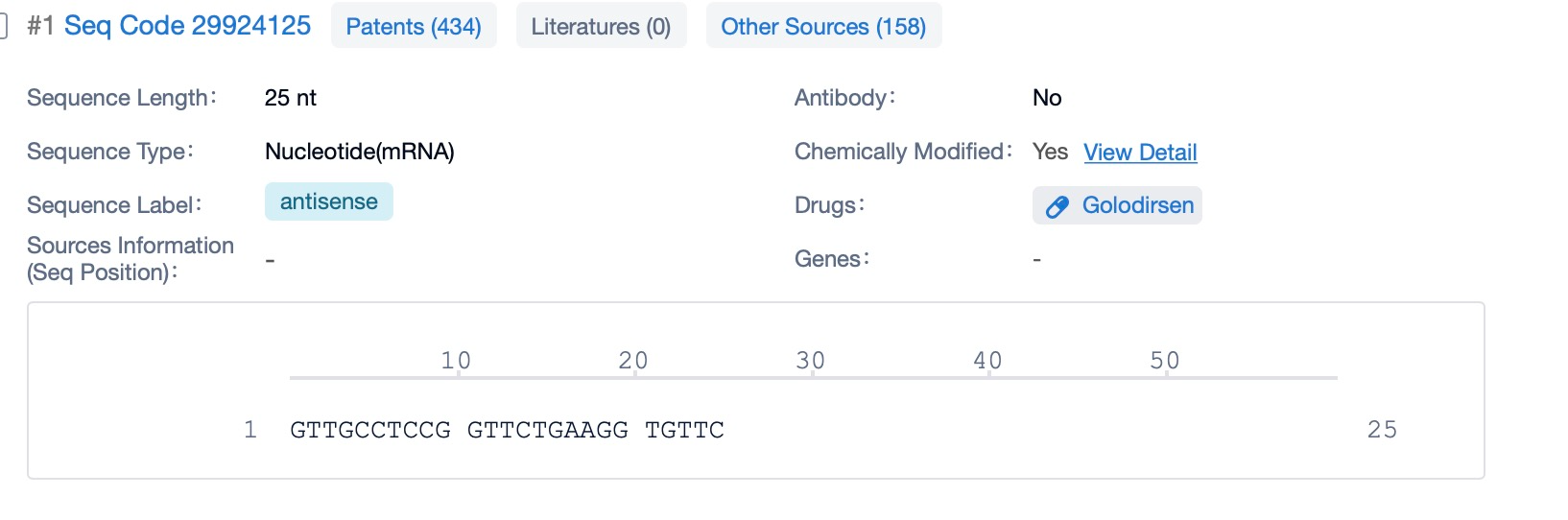
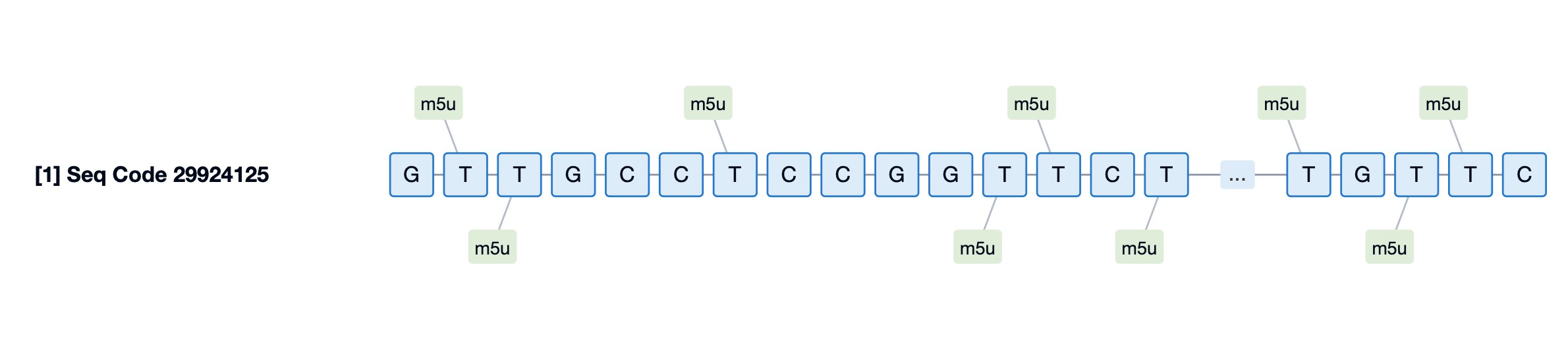
The PMO backbone of golodirsen contributes to its stability by replacing the natural phosphodiester bonds found in DNA with phosphorodiamidate linkages. These modifications make the molecule more resistant to nucleases, ensuring that it remains intact until it reaches its target within the cell. The specific sequence of golodirsen is optimized to have high affinity and specificity for its intended binding site on the pre-mRNA, which is critical for the efficiency and selectivity of exon skipping.
This targeted sequence design allows golodirsen to precisely alter the splicing of the DMD gene transcript, promoting the inclusion of sequences that can be translated into a functional dystrophin protein, despite the presence of mutations that would otherwise disrupt the reading frame. The resulting dystrophin protein, though shortened, retains enough of its critical domains to provide some level of functional restoration, thereby potentially mitigating the symptoms of Duchenne muscular dystrophy (DMD) in patients whose mutations are amenable to exon 53 skipping.
Chemical Modification and Species of Golodirsen
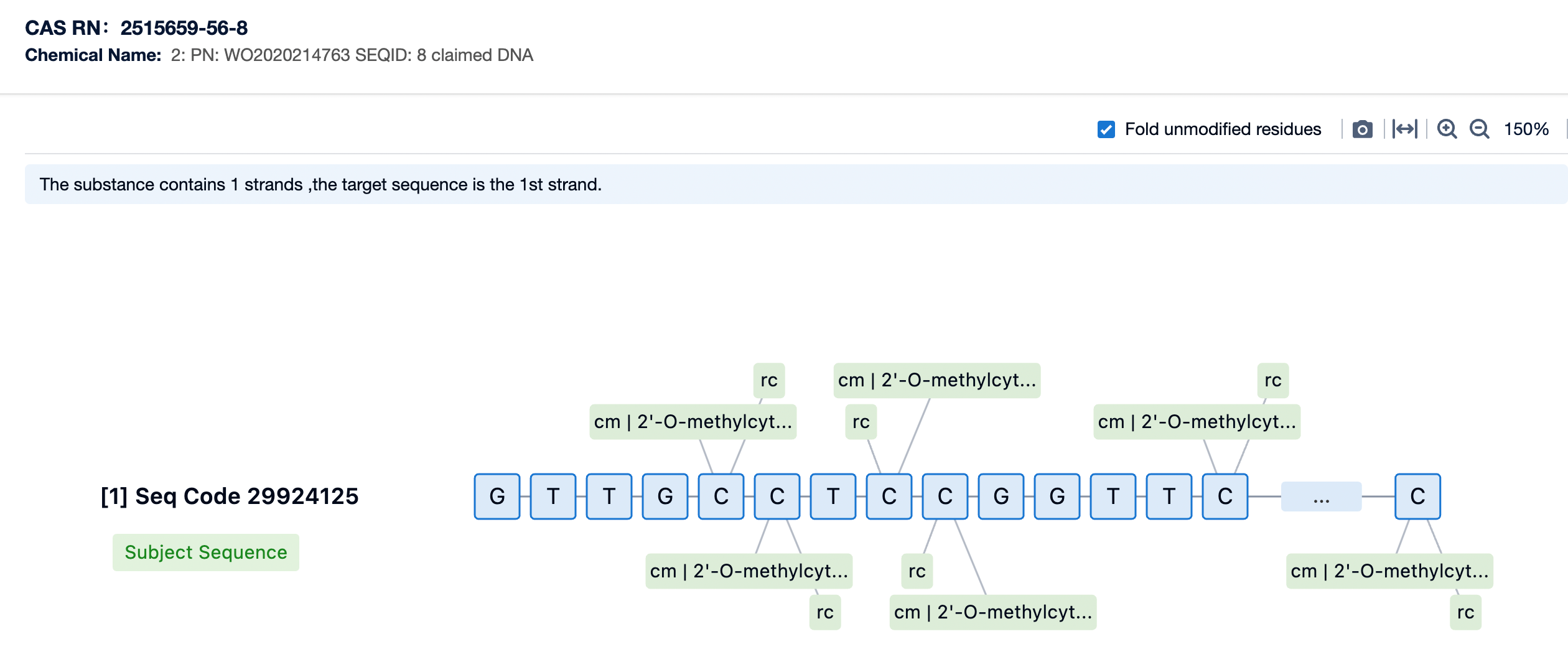
The chemical modifications of golodirsen include the use of morpholino rings, which replace the natural deoxyribose sugar-phosphate backbone found in DNA. This modification enhances the drug's resistance to exonuclease degradation, thereby increasing its stability and half-life in the body. Golodirsen also features a conjugation with a cell-penetrating peptide, which aids in its uptake by muscle cells.
One of the key modifications involves the substitution of natural deoxyribose sugar-phosphate backbones found in DNA with morpholino rings. These morpholino rings impart greater resistance to exonuclease degradation, significantly increasing the stability and half-life of the drug within the body. This modification is crucial for ensuring that golodirsen remains intact long enough to reach its target and perform its intended function of inducing exon skipping.
Furthermore, golodirsen is conjugated with a cell-penetrating peptide (CPP). This conjugation facilitates the efficient uptake of the oligonucleotide by muscle cells, overcoming the barrier posed by the cell membrane. The CPP acts as a carrier, helping to transport golodirsen across the cell membrane and into the cell's interior, where it can interact with the pre-mRNA and induce the necessary splicing changes. This enhancement is vital for the drug's effectiveness, as it ensures that golodirsen can reach the site of action and exert its therapeutic effects.
Together, these chemical modifications—morpholino rings for enhanced stability and a cell-penetrating peptide for improved cellular uptake—contribute to the overall design of golodirsen, optimizing its performance as a targeted therapy for DMD. These features enable golodirsen to effectively reach and modify the genetic material within muscle cells, promoting the production of a modified but functional dystrophin protein, which is essential for muscle health and function.
Summary and Prospect
In summary, golodirsen is a pioneering therapy for DMD that offers a targeted approach to addressing the underlying genetic cause of the disease. Its approval and ongoing clinical development represent a significant step forward in the treatment of DMD, particularly for patients with mutations amenable to exon 53 skipping. As clinical trials continue to evaluate its long-term safety and efficacy, golodirsen holds promise for improving outcomes and quality of life for individuals with DMD. The future of golodirsen may include further exploration of its potential in combination with other therapies, as well as the development of additional exon-skipping treatments to address the broader DMD patient population.
How to find the sequence of an ASO?
In Patsnap Bio, you can find the sequence and latest research and development advances of all ASOs.
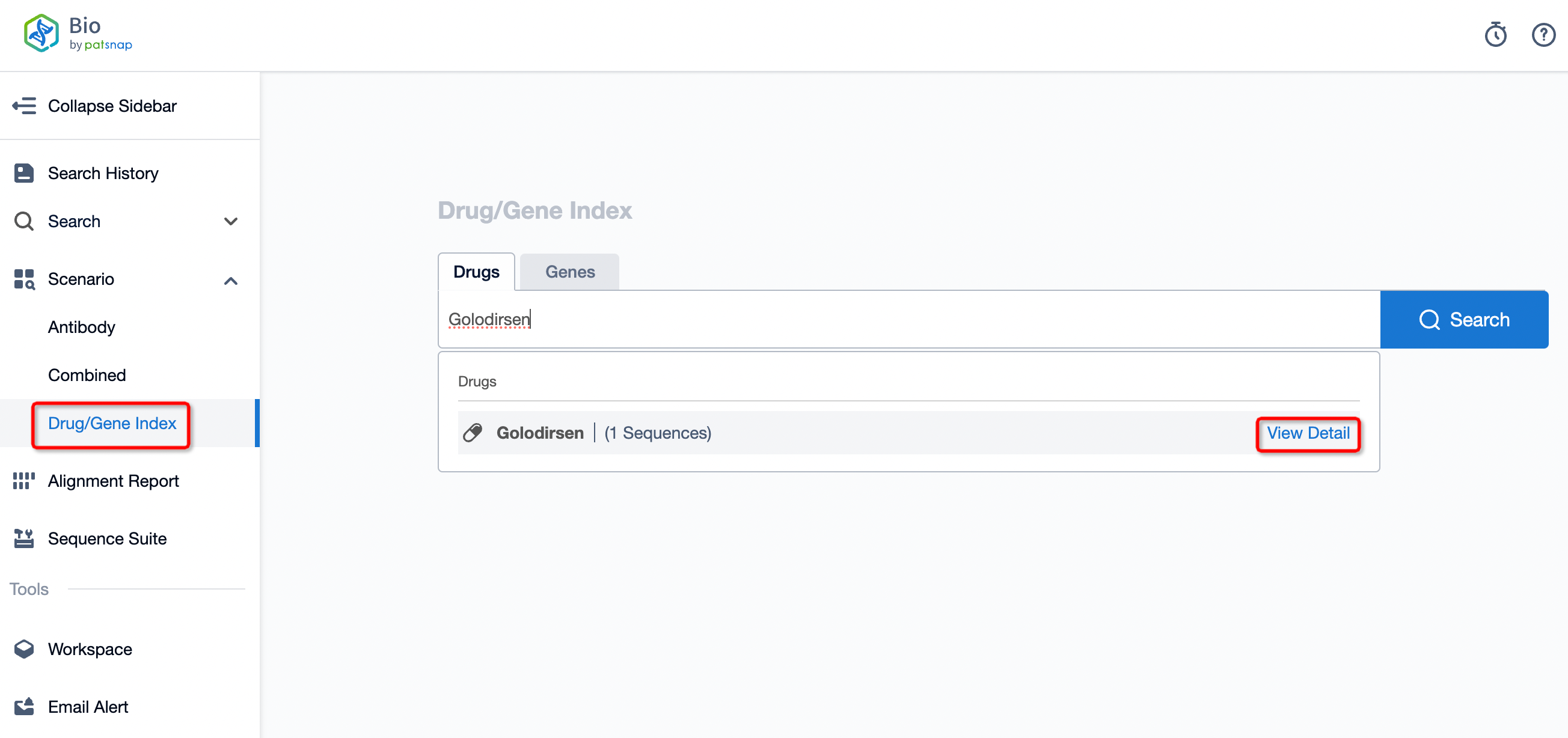
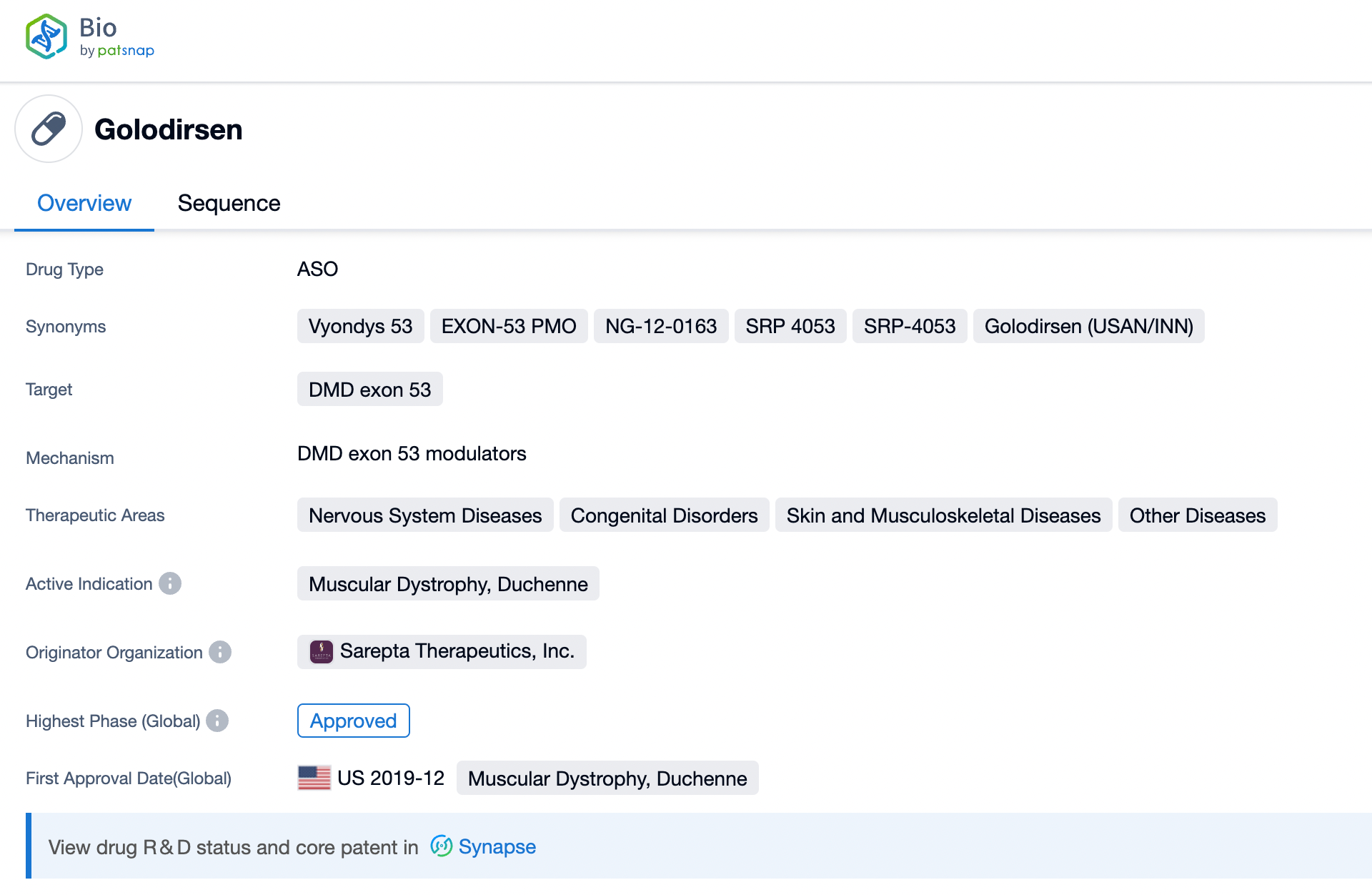
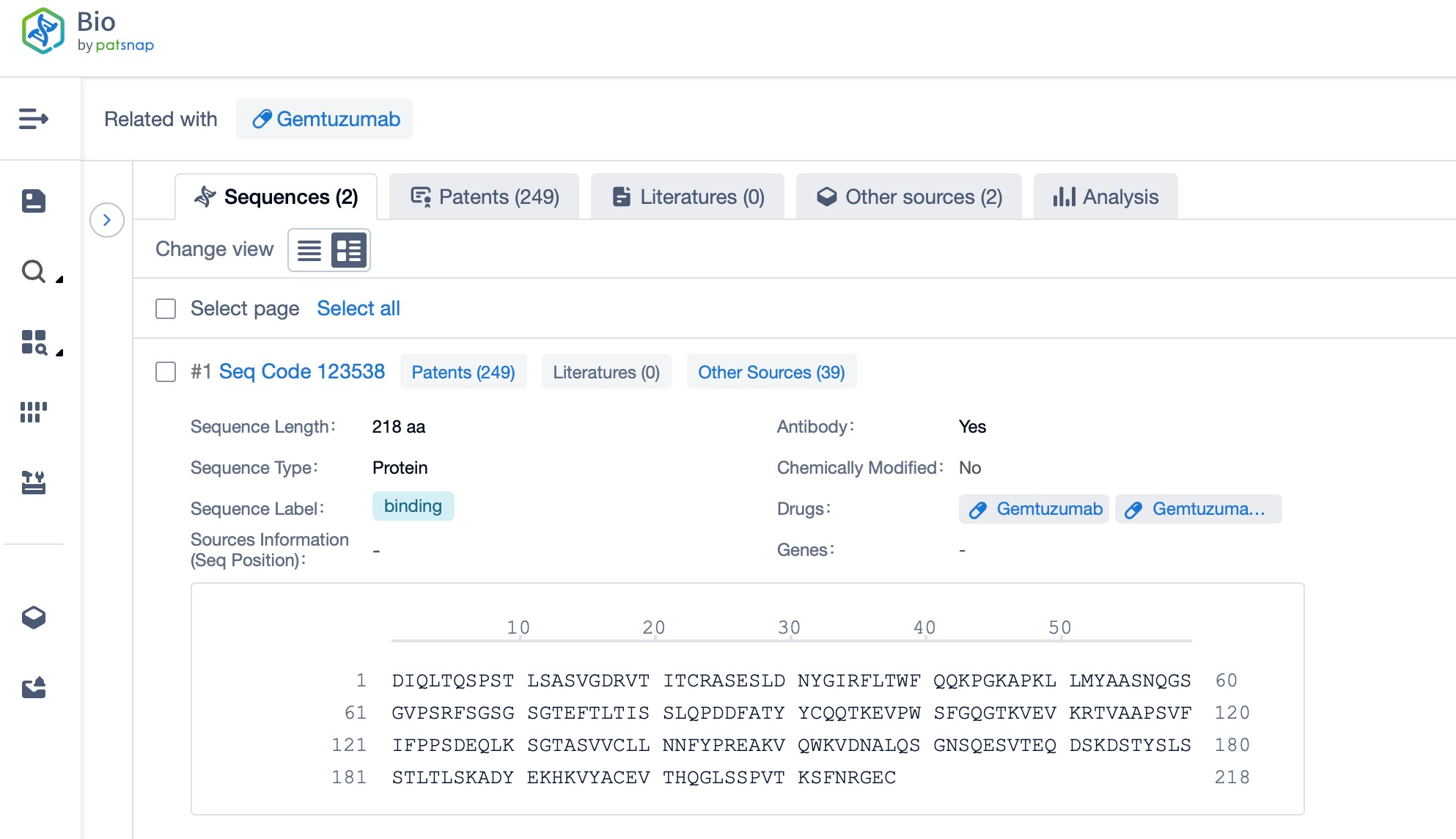
Taking golodirsen as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter 'golodirsen' in the search box and click to view the details. On the details page, you can find the basic information and research progress of golodirsen.
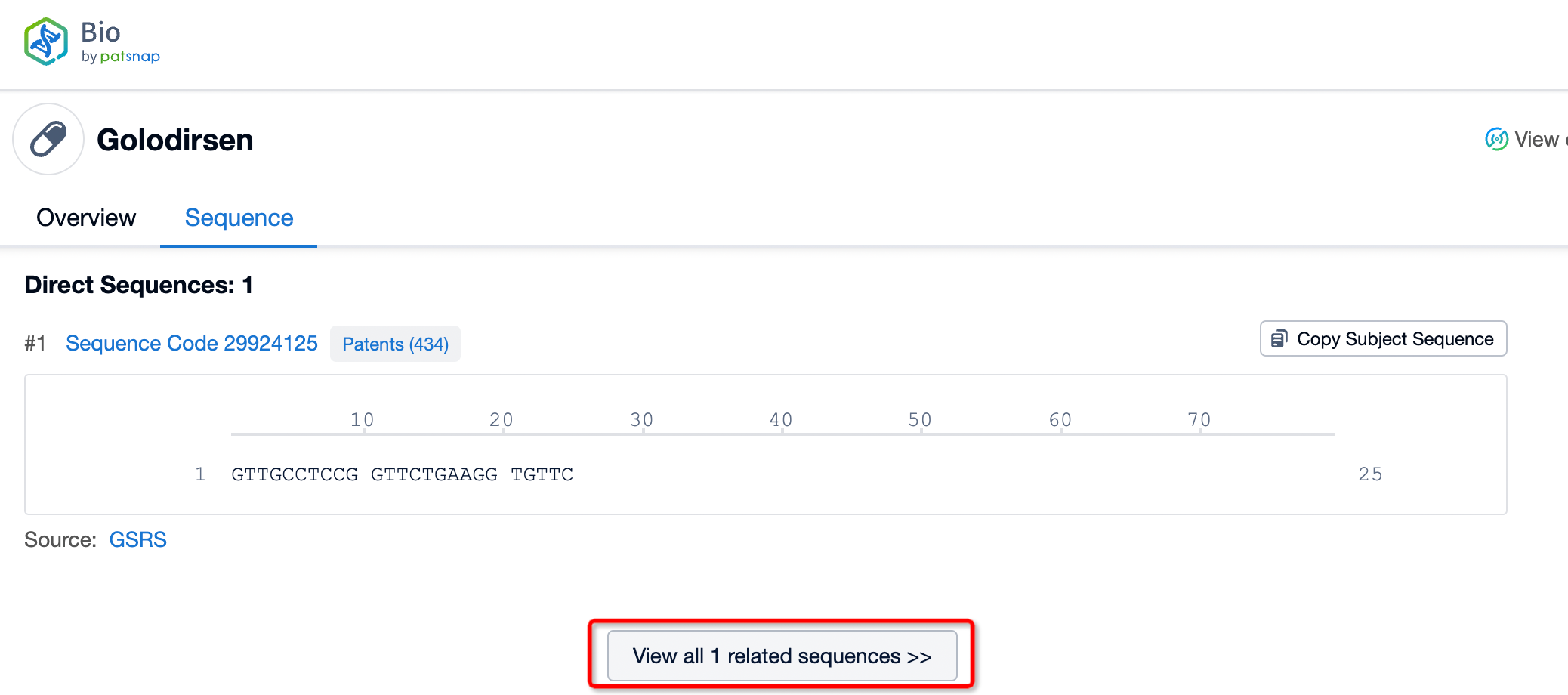
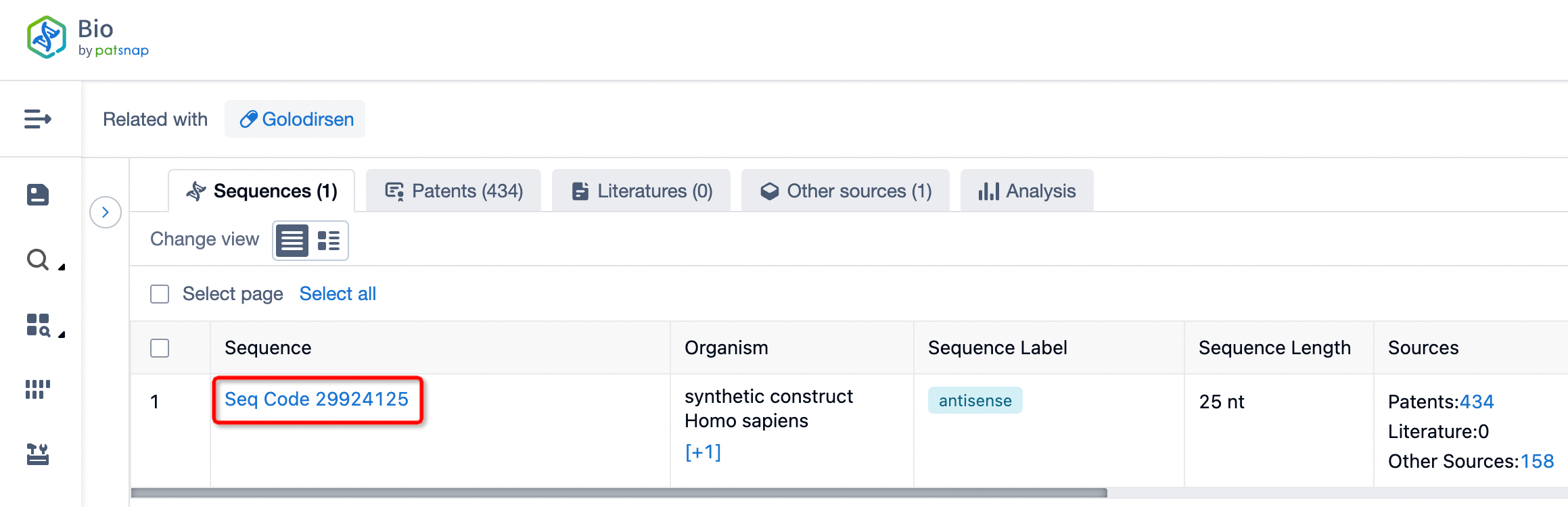
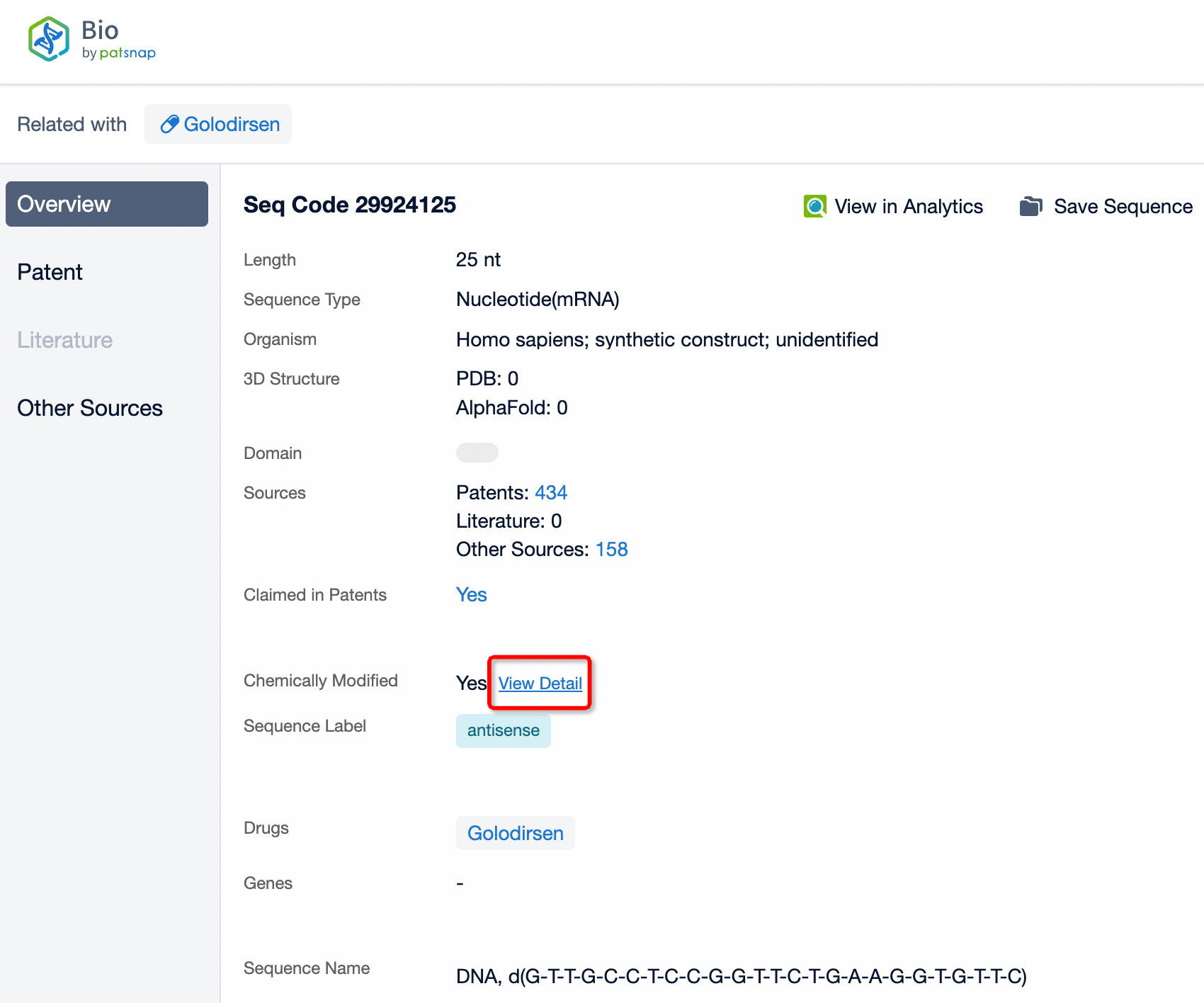
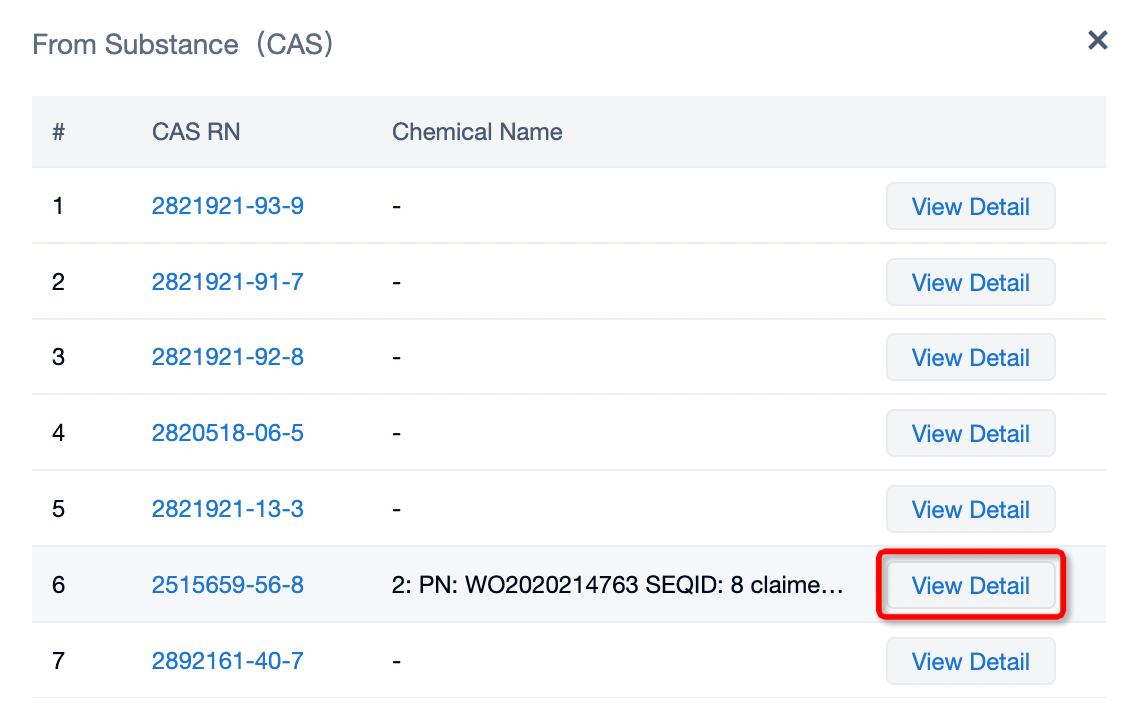
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
Clicking on the sequence name will provide you with all the basic information of that sequence.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.