Phase 2/3 Study Shows Nipocalimab Effectively Controls Symptoms in Teenagers with Generalized Myasthenia Gravis
Johnson & Johnson (NYSE: JNJ) revealed favorable outcomes from the Phase 2/3 Vibrance-MG trial assessing nipocalimab in adolescents (12-17 years) with generalized myasthenia gravis (gMG) who are anti-AChRa positive. Those who received both nipocalimab and the standard of care (SOC) experienced consistent disease management, demonstrated by the main goal of reducing immunoglobulin G (IgG) levels from the starting point over 24 weeks. Additionally, there were enhancements in MG-ADLb and QMGc scores as secondary measurements.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The data from the Phase 2/3 study will be highlighted in an oral presentation (Abstract #MG100) at the Myasthenia Gravis Foundation of America (MGFA) Scientific Session during the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) Annual Meeting. At this event, Johnson & Johnson will present 25 abstracts.
"The results from the Vibrance-MG study emphasize the promise of this investigational therapy for adolescents aged 12-17 years with generalized Myasthenia Gravis (gMG). The data indicate a substantial decrease in Immunoglobulin G (IgG) levels, approximately 70%, in the adolescent group, along with clinical benefits aligning with the Vivacity-MG3 findings in adults," explained Jonathan Strober, M.D., Director of Clinical Services for Child Neurology and the Muscular Dystrophy Clinic at UCSF Benioff Children’s Hospital. "These positive outcomes are encouraging, especially since there are no approved advanced treatment options currently available for this age group in the United States."
Around 10% of new myasthenia gravis cases are identified in adolescents (aged 12-17). The severity of gMG in pediatric patients is notable, with 43% experiencing more than five hospitalizations, 46% requiring at least one intensive care unit admission, and 68% facing periods of disease exacerbation.
The treatment regimen combining nipocalimab with standard of care successfully achieved the study's primary endpoint, demonstrating a 69% reduction in total serum IgG. It also met secondary endpoints that assessed disease activity using MG-ADL and QMG. By the end of treatment, four out of five patients reached minimal symptom expression, achieving MG-ADL scores of 0-1. Nipocalimab was generally well-tolerated over a six-month period, similar to the tolerability observed in adult participants in the Vivacity-MG3 study, with no serious adverse events reported and no treatment discontinuations due to adverse effects.
These open-label Phase 2/3 results in teenagers are, for the first time, showing consistency with findings from the pivotal study of nipocalimab in adults with gMG. Nipocalimab, when added to standard care, is the first FcRn blocker to demonstrate sustained disease control in a registrational trial, as indicated by improvements in MG-ADL compared to placebo plus standard care over six months of consistent dosing (every two weeks) in adults with gMG.
"The Vibrance-MG findings broaden the clinical understanding of nipocalimab and highlight its potential impact on adolescents with gMG who need new therapeutic options," stated Sindhu Ramchandren, M.D., Executive Medical Director, Neuroscience, Johnson & Johnson Innovative Medicine. "Our commitment is to create innovative solutions for autoimmune-driven neurological disorders, like gMG, with the goal of transforming the lives of those affected by these conditions."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
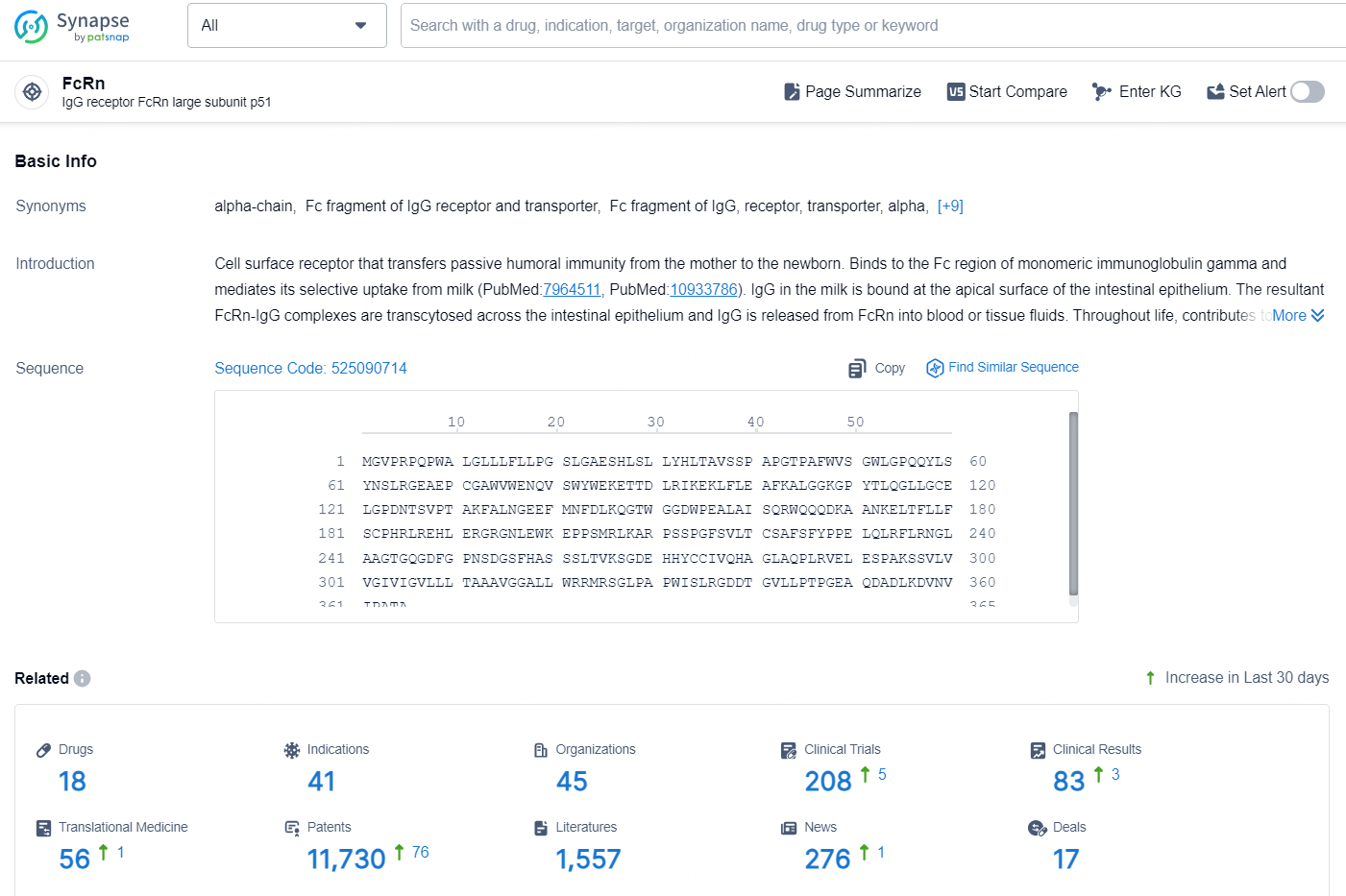
According to the data provided by the Synapse Database, As of October 16, 2024, there are 18 investigational drug for the FcRn targets, including 41 indications, 45 R&D institutions involved, with related clinical trials reaching 208, and as many as 11730 patents.
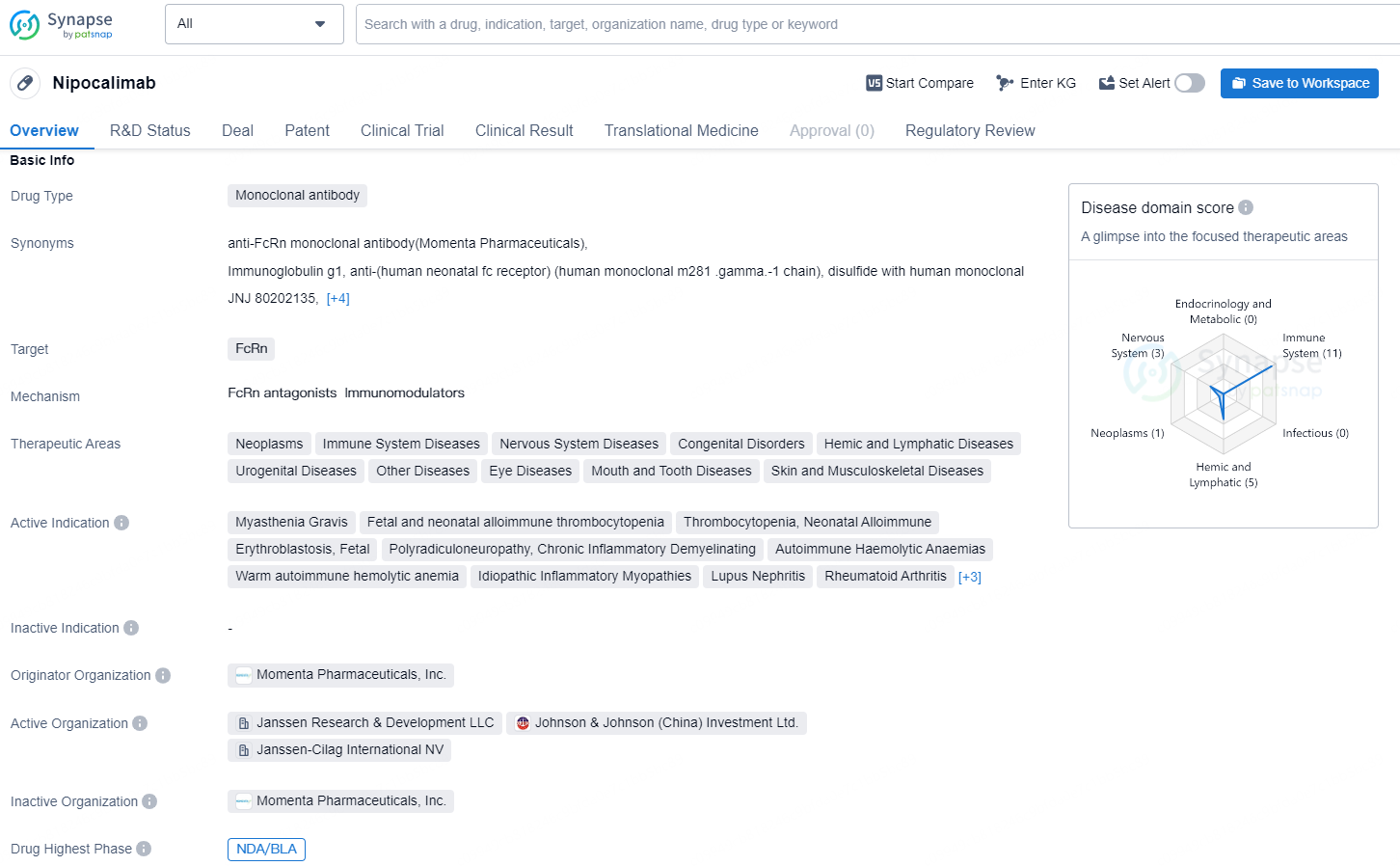
Nipocalimab is a monoclonal antibody drug developed by Momenta Pharmaceuticals, Inc. that targets the FcRn protein. The drug is being developed to treat a wide range of therapeutic areas including neoplasms, immune system diseases, nervous system diseases, congenital disorders, hemic and lymphatic diseases, urogenital diseases, eye diseases, mouth and tooth diseases, and skin and musculoskeletal diseases.