Topas Therapeutics Reports Positive Phase 2a Trial Results for TPM502 in Celiac Disease Patients
Topas Therapeutics released encouraging top-line outcomes from a Phase 2a study examining their leading candidate, TPM502, in individuals with celiac disease. The results provide initial clinical evidence for Topas’ unique nanoparticle platform and its capability to promote targeted, antigen-specific tolerance effects.
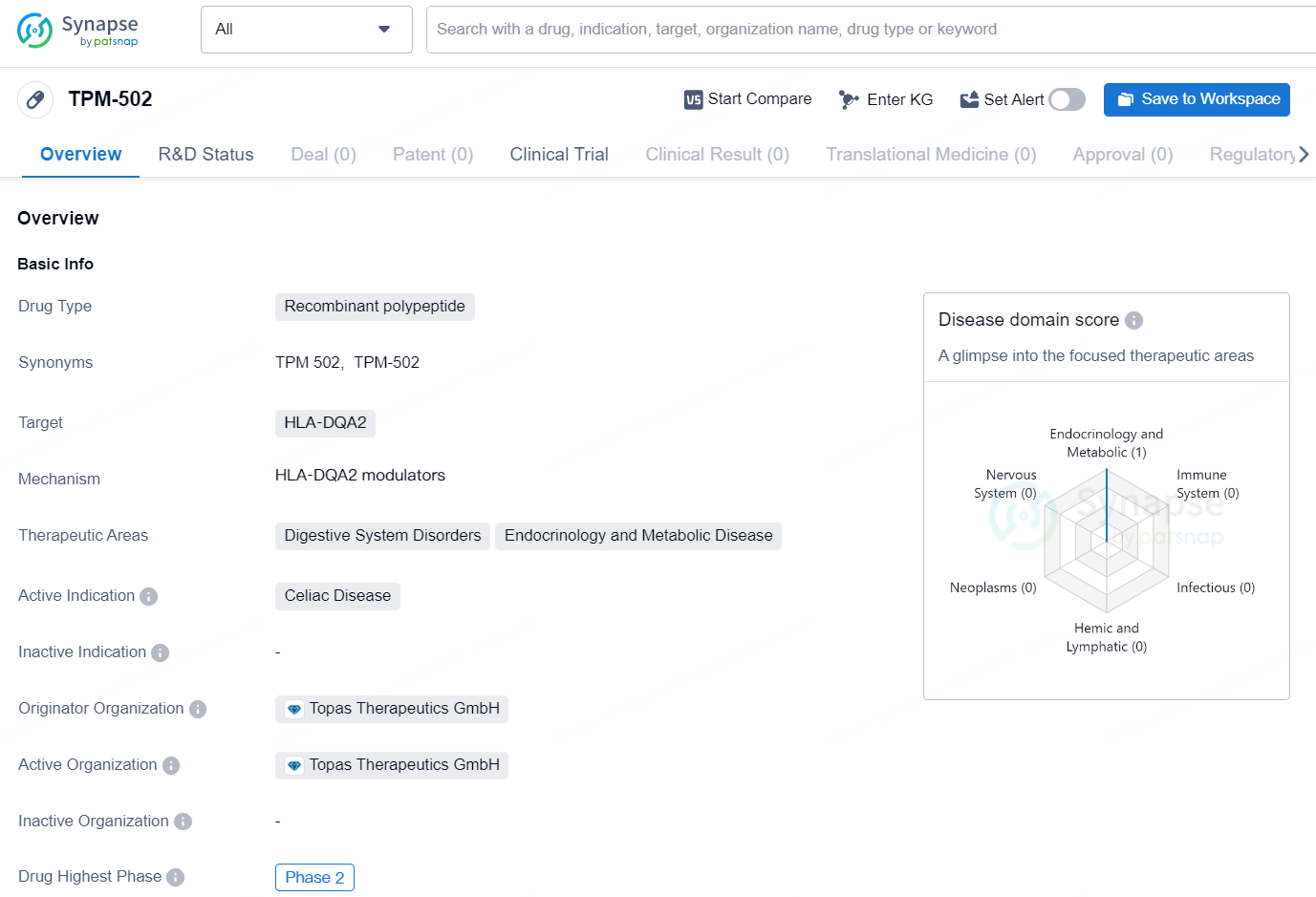
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In 2023, a global, multi-center, double-blind, randomized, placebo-controlled Phase 2a study (NCT05660109) commenced to explore the safety, tolerability, and pharmacodynamics of administering two doses of TPM502 to adults with celiac disease. The pharmacodynamic outcomes were evaluated through a gluten challenge after subjects received either TPM502 or a placebo. Initial findings indicated a significant, dose-dependent response in antigen-specific markers for tolerance induction. Additionally, these effects were sustained throughout the study's follow-up duration. TPM502 was deemed safe across all dosage levels tested. Topas plans to present the complete data and analysis at an upcoming scientific conference and intends to publish the results in a peer-reviewed journal.
"The initial insights from the TPM502 Phase 2a data mark a significant achievement for the Topas team, reinforcing our goal to prove the antigen-specific tolerogenic effects of our platform. The data from this trial emphasize the potential of this innovative, flexible approach in treating a wide range of autoimmune and immune-mediated conditions," stated Hugo Fry, CEO of Topas Therapeutics. "These findings provide a strong foundation for the subsequent phase of Topas' development and strategically position the company within the field of immune tolerance."
Topas' leading candidate, TPM502, utilizes the company's advanced platform, which is a nanotechnology-based strategy designed to provoke selective, antigen-specific immune tolerance. TPM502 consists of nanoparticles encapsulating the primary gluten epitopes for HLA-DQ2.5, which are present in most celiac disease patients. It aims to create durable immune tolerance, offering substantial therapeutic advantages for celiac patients who currently lack treatment options.
The launch of the Phase 2a trial for TPM502 is based on encouraging preclinical results and the excellent safety record shown in the Phase 1 trial that evaluated Topas' TPM203 in patients with pemphigus vulgaris. The next steps in the development of TPM502 will depend on the comprehensive analysis of the study data.
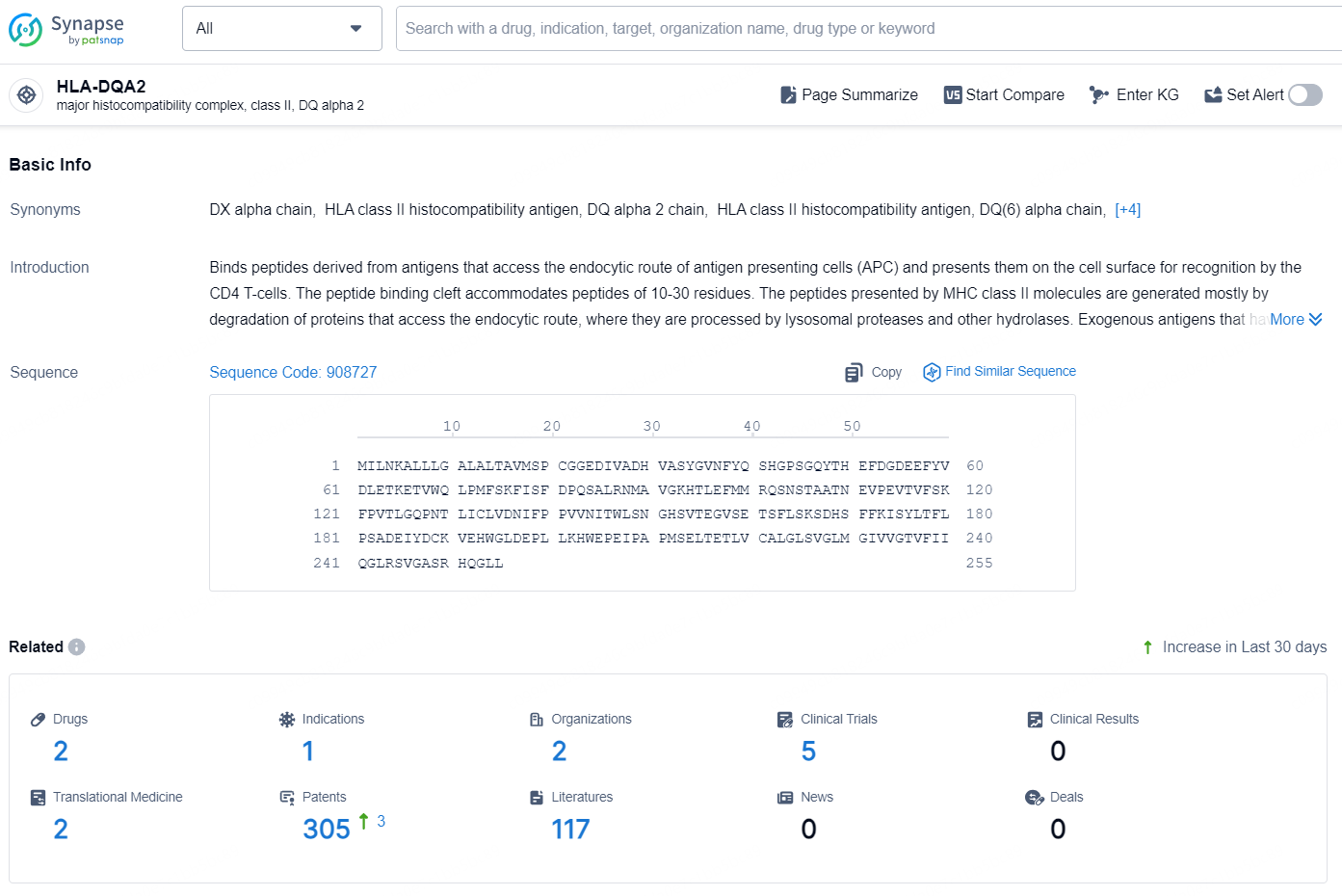
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 16, 2024, there are 2 investigational drug for the HLA-DQA2 target, including 1 indication, 2 R&D institutions involved, with related clinical trials reaching 5, and as many as 305 patents.
The drug TPM-502 is a recombinant polypeptide that targets HLA-DQA2. It is indicated for the treatment of Celiac Disease and falls within the therapeutic areas of Digestive System Disorders, Endocrinology, and Metabolic Disease. The drug is currently at the highest phase of development, which is Phase 2, on a global scale.