Hunting Fungal Superbugs: Crafting New Xanthone Weapons Against Deadly Drug-Resistant Infections
Fungal infections can cause severe and sometimes fatal conditions, especially in people with weakened immune systems. This includes individuals with immunodeficiency or those receiving immunosuppressive treatments like transplant recipients.
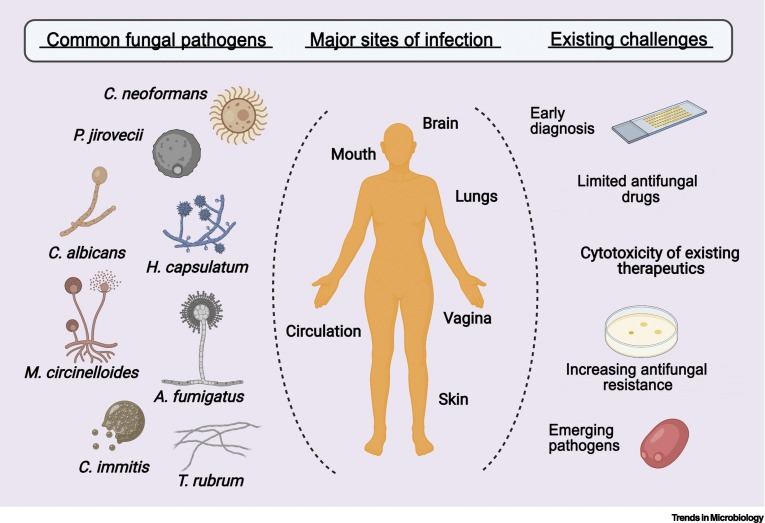
In fact, fungi are among the most common causes of healthcare-associated infections (HAIs). For instance, Candida albicans is the fourth most prevalent pathogen worldwide responsible for nasal infections. Additionally, chemotherapies used in clinical care can spur the rise of drug-resistant fungi.
Statistics show over 1.6 million people die annually from fungal infections and related complications - comparable to tuberculosis deaths. Yeast-like Candida albicans and Candida glabrata, as well as filamentous fungi from the Aspergillus genus, are primary causes of systemic fungal diseases for susceptible individuals. The emergence of multidrug-resistant Candida fungi, such as Candida auris, also presents a major global health challenge.
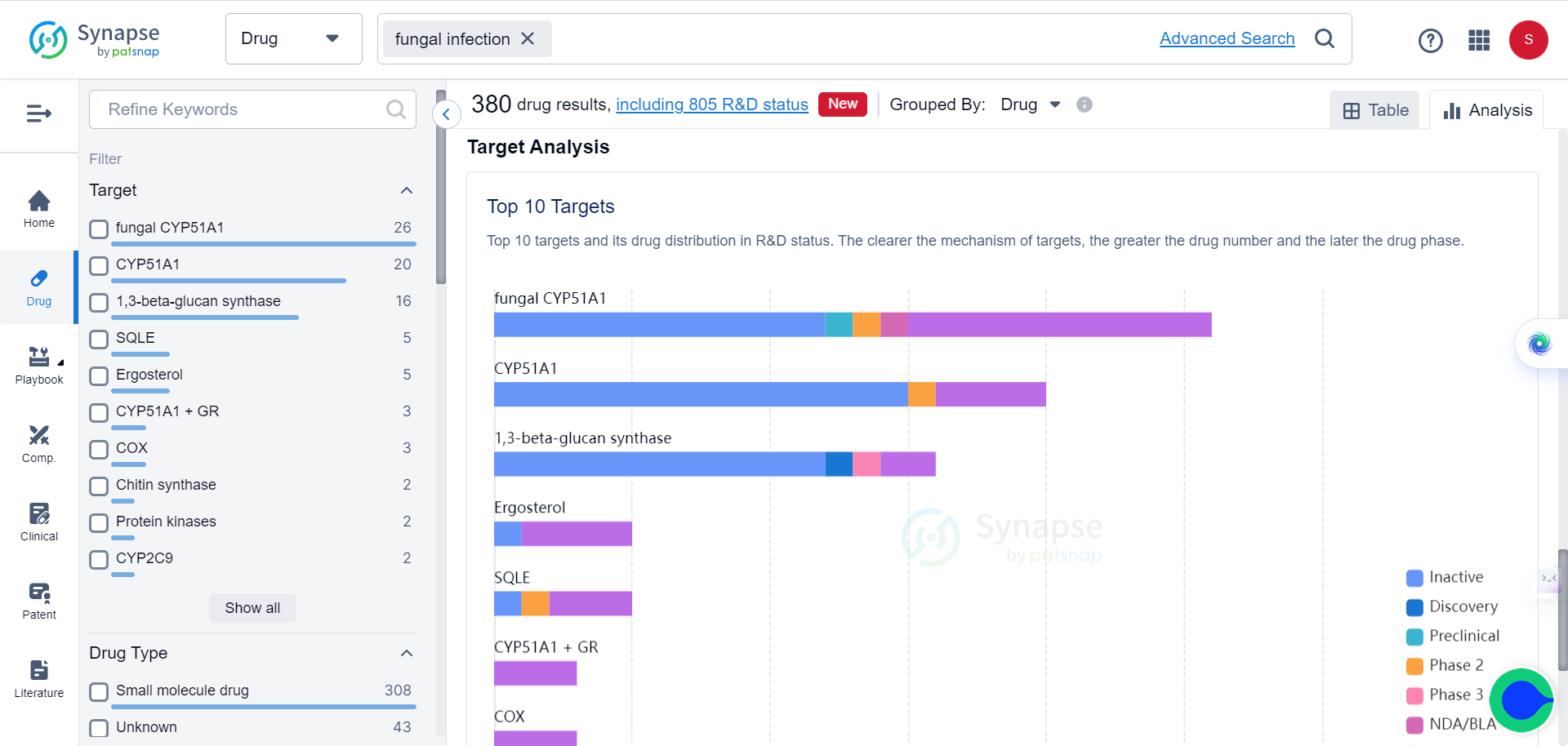
Synthesis of xanthone derivatives
A recent research paper titled "Xanthone Synthetic Derivatives with High Anticandidal Activity and Positive Mycostatic Selectivity Index Values," published in Scientific Reports, presented the synthesis and evaluation of 28 derivatives of xanthone and benzoxanthone. These analogues were tested for their antifungal activities, further underscoring the potential of xanthones as antifungal drugs.
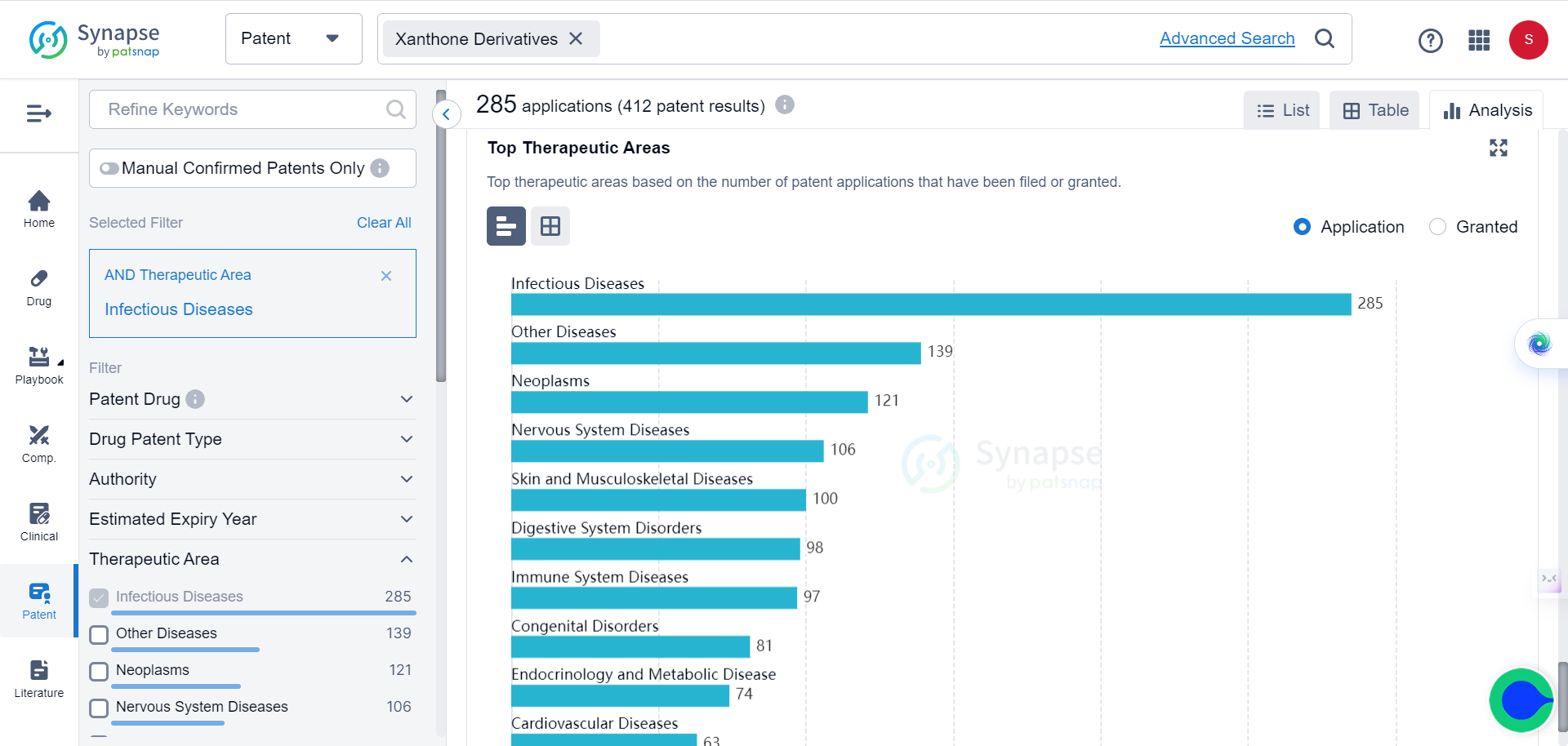
According to Synapse database, among the 412 patents granted to Xanthone derivatives, 285 applications (the most) are intended for infectious diseases, which include fungi infections.
The researchers were primarily interested in creating new antifungal drugs aimed at inhibiting fungal topoisomerases. Prior in-depth studies on the structure and function of fungal topoisomerase I and II have demonstrated their pivotal role in certain strains. Additionally, the inhibition of yeast topoisomerase II can elicit antifungal activity, and potentially overcome resistance to fluconazole.

The researchers designed their antifungal drugs around xanthone derivatives. These natural derivatives, found as metabolites in a variety of plants, lichens, fungi, and bacteria, are a promising class of antifungal compounds. Their intriguing structural scaffolds and bioactivities have encouraged many scientists to create xanthone derivatives, aiming to develop new promising drug candidates with potential therapeutic properties against cancer, microbes, malarial, HIV, oxidative stress, and inflammation.
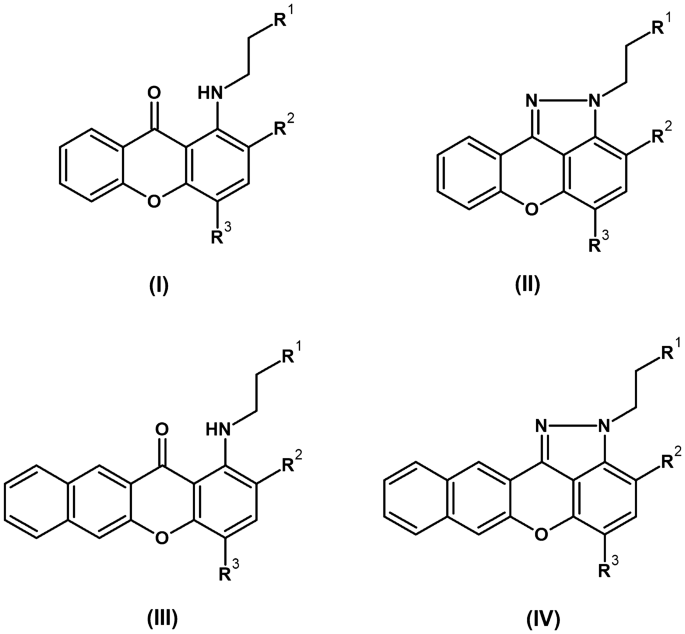
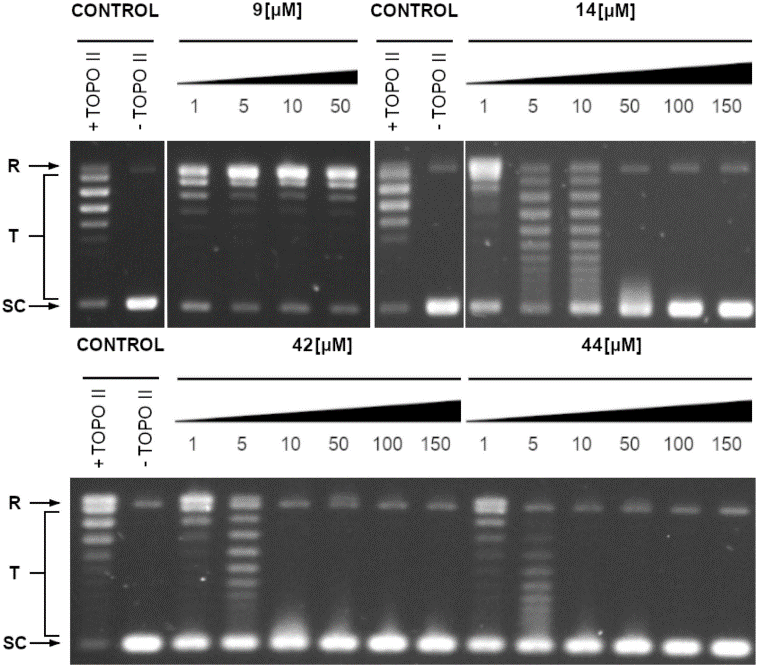
Despite the increasing interest in these compounds, there have been few detailed reports exploring their antifungal mechanism of action. Drawing on previous studies that identified xanthone analogues as potential antibacterials, the researchers decided to examine the antifungal activity of four new groups of compounds and their inhibitory effects on yeast topoisomerase II (yTOPO II).
In this study, four groups comprising 28 xanthone derivatives were synthesized. Compounds 1 – 16 and 34 were prepared using previously published procedures, while compounds 25, 26, 35-38, and 41-45 are newly synthesized analogues. The four groups (I-IV) are differentiated by nitro or amino substitution, and the presence of a pyrazole or a benzofused ring on the xanthone core. A structure-activity relationship analysis revealed that a naphthalene ring and a nitro group at position R3 are critical for antifungal activity. Consequently, the researchers synthesized more potent derivatives by modifying these groups in the initial derivatives. For instance, derivatives 35-38 and 41-45 were synthesized from compound 9, yielding derivative 44, which showed improved potency against the reference strains compared to the original derivative 9.
Overcome the drug resistance
The researchers conducted drug susceptibility testing of these new compounds against various fungal strains. They evaluated the in vitro antifungal activity of all 28 derivatives against five reference fungal strains from the American Type Culture Collection (ATCC). The 13 most active compounds were then tested against six clinical isolates of Candida albicans, both fluconazole-susceptible (B3, Gu4, and F2) and -resistant (B4, Gu5, and F5). The minimum inhibitory concentrations (MICs) of the test compounds were determined using microdilution assays.
The researchers also delved into the molecular mechanism underlying the antifungal activity of these derivatives. They meticulously determined the fungicidal activity and minimum fungicidal concentrations (MFCs) of carefully selected derivatives. The resulting biological profile strongly suggested that the most potent derivatives functioned effectively as fungicides, thereby increasing the likelihood of successful early treatment against invasive candidiasis and candidemia. This, in turn, reduces the risk of persistent or recurrent infections and the development of drug resistance.
Furthermore, the study extensively explored the selectivity of these novel compounds in relation to mammalian cells. Human embryonic kidney cell line (HEK-293) and human hepatocellular carcinoma cell line (HEPG2) were employed, and the results were compared with previously determined cytotoxic activity against the human colon adenocarcinoma cell line (HT29). The newly synthesized compounds exhibited acceptable levels of cytotoxicity. Notably, benzoxanthones emerged as the leading antifungal agents, displaying positive mycostatic selectivity index values for both the HEK293 and HEPG2 cell lines. The presence of a fused benzene ring was identified as a critical factor contributing to their potent activity.
Given that xanthone derivatives are inhibitors of human topoisomerase, the antifungal activity of the analyzed compounds could be associated with their inhibitory effect on the fungal equivalent of this enzyme. The results of the study demonstrated a close relationship between antifungal activity and the inhibition of yeast topoisomerase II.
The study provided proof of concept that by modifying xanthones, a new series of selective antifungal drugs targeting fungal topoisomerase II can be discovered. Furthermore, the results suggested that these derivatives could be used to combat drug-resistant fungal cells. This further validates the potential of xanthones as antifungal drugs. Future research will focus on optimizing their selectivity.

Reference
Rząd, K., Ioannidi, R., Marakos, P. et al. Xanthone synthetic derivatives with high anticandidal activity and positive mycostatic selectivity index values. Sci Rep 13, 11893 (2023).