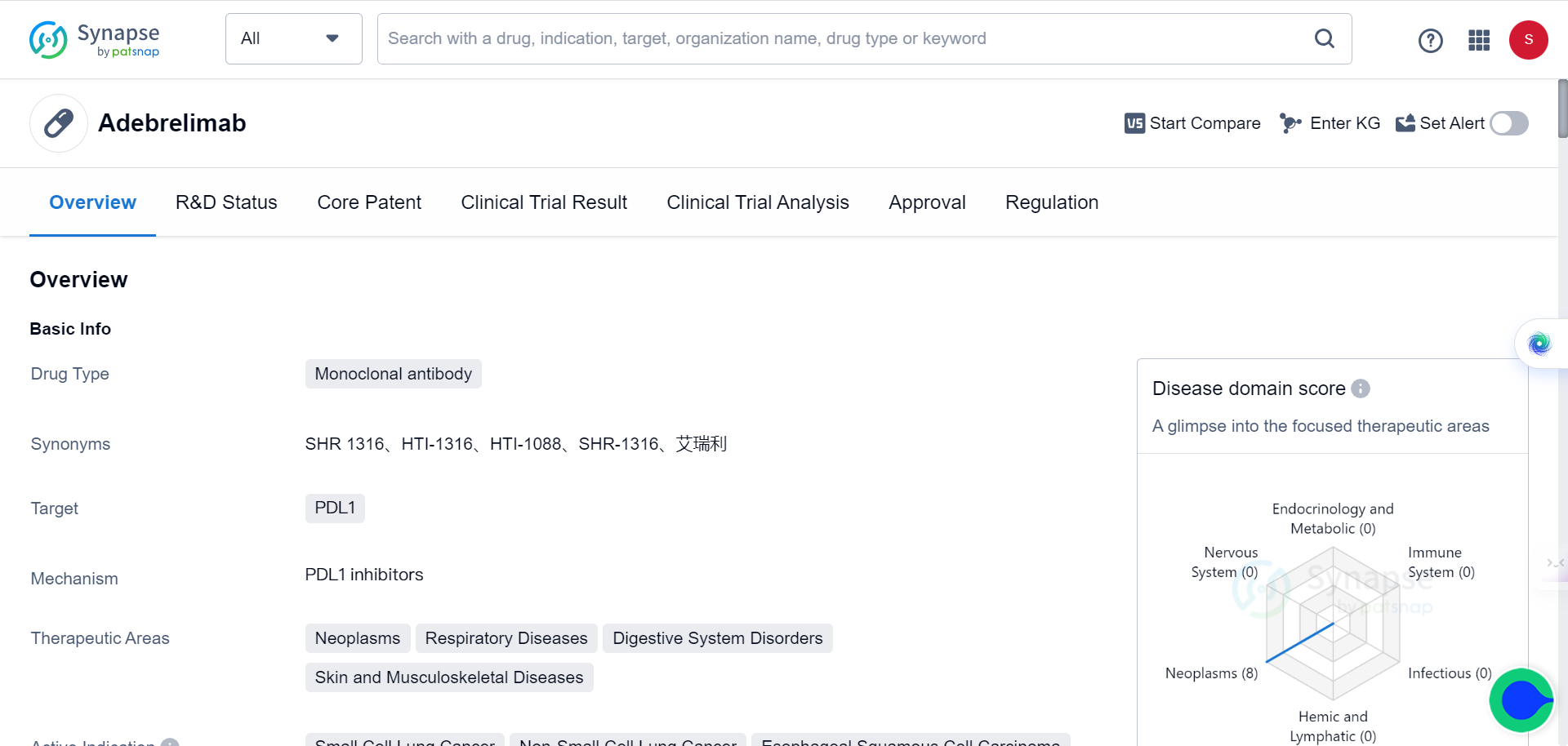
The First Phase 1b Trial Shows Promising Results of Neoadjuvant Adebrelimab for Esophageal Squamous Cell Carcinoma
The Chinese Academy of Sciences' Center for Systems Biology recently published a research article in Nature Medicine titled "Neoadjuvant Adebrelimab in Locally Advanced Resectable Esophageal Squamous Cell Carcinoma: A Phase 1b Trial," which reports on the results of the world’s first-ever neoadjuvant mono-immunotherapy Phase 1b clinical trial of locally advanced esophageal squamous cell carcinoma. The study proposes new strategies for predicting tumor immunotherapy efficacy based on multi-omics studies.
Neoadjuvant immunotherapy
Esophageal cancer is one of the most prevalent malignant tumors of the digestive tract worldwide, with over 90% of cases comprising esophageal squamous cell carcinomas (ESCC), representing nearly 60% of new cases globally. It ranks fourth among common cancers in terms of mortality. Unfortunately, most patients with esophageal cancer are in the locally advanced stage at the time of diagnosis. The current standard clinical treatment methods are neoadjuvant chemoradiotherapy or chemotherapy after surgery. However, these treatments have limited efficacy, leading to local recurrence or distant metastasis and ultimately compromising patient survival rates.
Recent years have seen the emergence of immunotherapy, represented by checkpoint inhibitors, which has demonstrated positive therapeutic effects in advanced solid tumors. Its application has gradually extended from second-line maintenance treatment to neoadjuvant treatment. However, the safety and benefits of neoadjuvant mono-immunotherapy for esophageal squamous cell carcinoma have not yet been reported, and there is no clear biological marker for predicting the efficacy of such immunotherapy.
To explore the safety and effectiveness of neoadjuvant mono-immunotherapy for patients with locally advanced resectable esophageal squamous cell carcinoma, the researchers carried out a prospective, single-center, single-arm, nonrandomized Phase 1b clinical trial (NATION-1907, registered number NCT04215471).
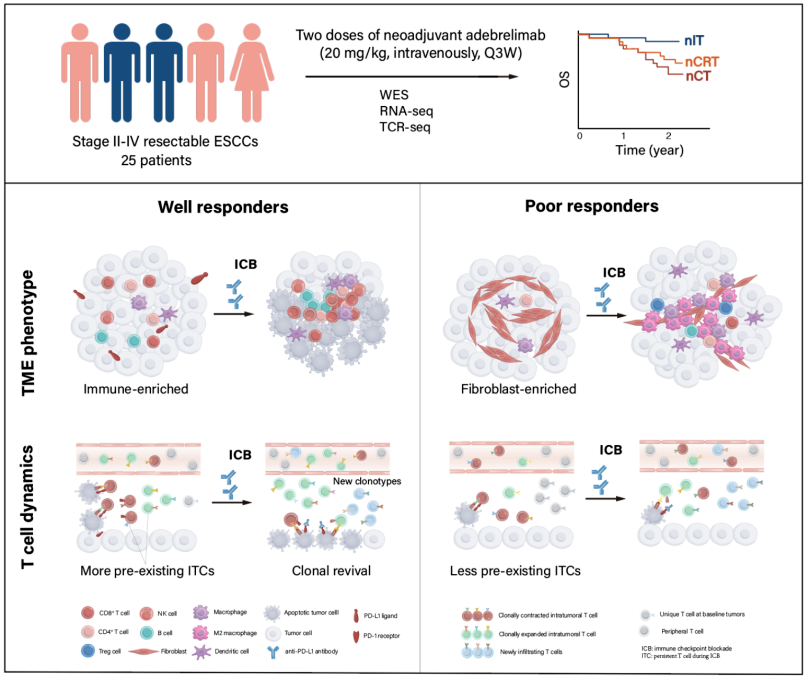
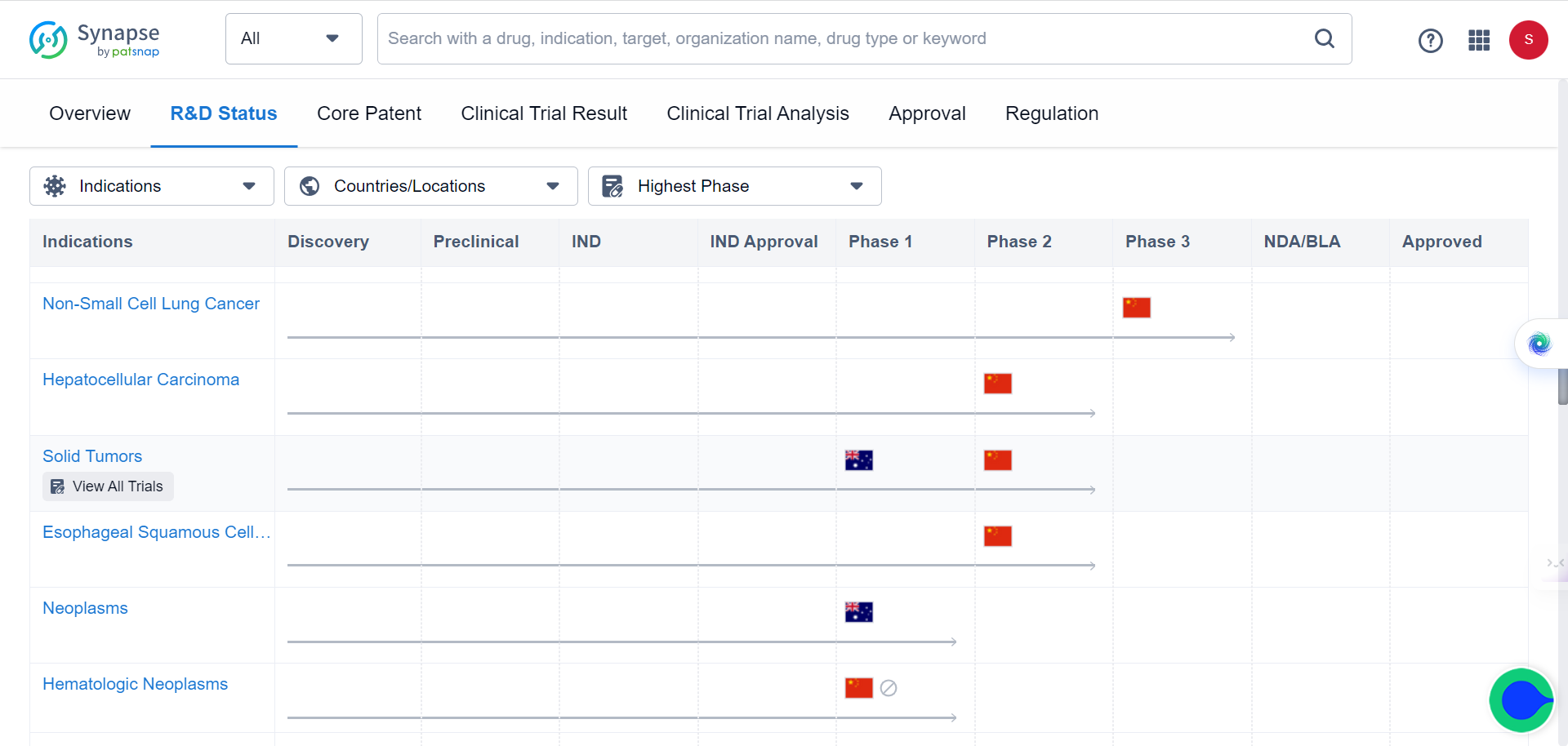
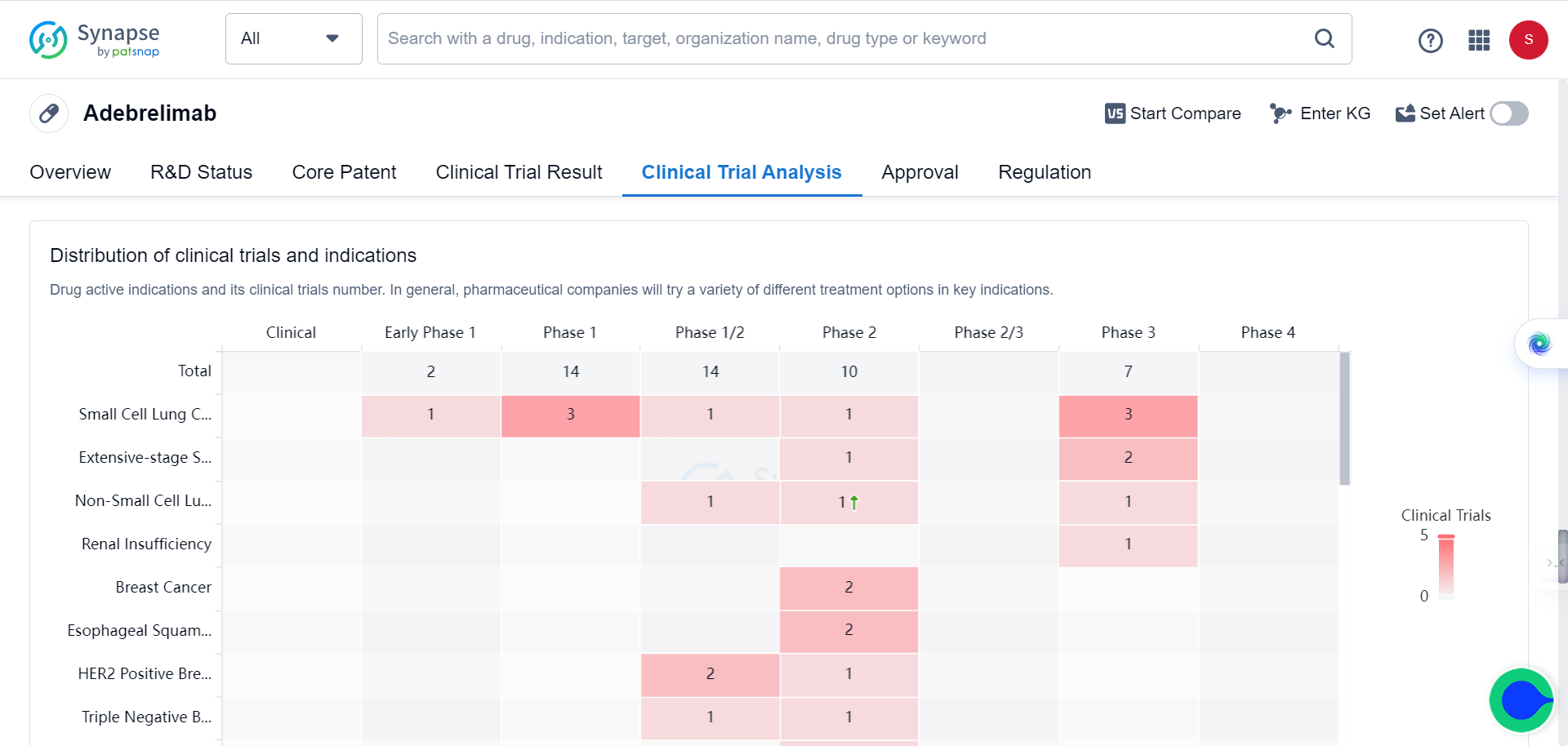
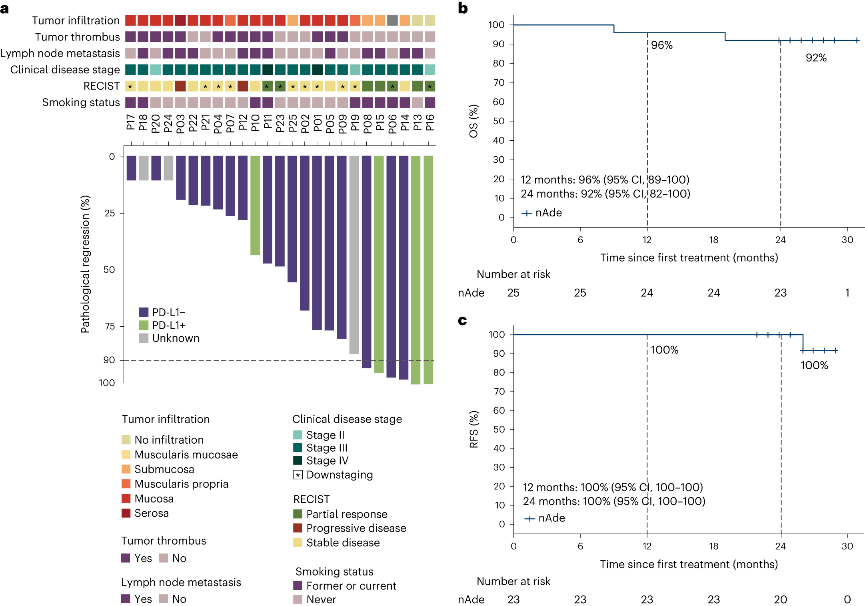
30 patients with stage II-IV resectable esophageal squamous cell carcinoma who underwent surgery after neoadjuvant treatment with Adebrelimab (anti-PD-L1 antibody) were enrolled for the study. Based on assessments of safety and effectiveness, the rate of major pathological response (MPR, tumor shrinkage ≥90%) and overall survival (OS) were elicited. Tumor tissues and blood samples of enrolled patients before and after treatment were collected to integrate genome and transcriptome sequencing, TCR sequencing, and multiple immunofluorescence techniques. The study compared the different molecular characteristics of the sensitive and insensitive groups to explore the potential mechanisms of treatment response and resistance.
Safety, efficacy and biomarkers
In the safety evaluation of the study, no adverse reactions over Grade 3 were observed, and 24% of patients achieved major pathological remission (MPR), with 2-year overall survival (OS) at 92% and 2-year disease-free survival (RFS) at 100%. Both treatment adverse reactions and 2-year survival status assessment demonstrated that the neoadjuvant anti-PD-L1 monotherapy adopted in this study was significantly better than neoadjuvant chemotherapy or chemoradiotherapy (compared with CMISG1701 clinical study), showing good safety and efficacy of the treatment regimen.
To compare well responders and poor responders, the research team further analyzed potential biomarkers associated with efficacy stratification. Although no significantly correlated mutation features were identified at the genome variation level, the researchers constructed an "IFN/EMT score" consisting of 12 characteristic genes based on transcriptome data. The score could significantly distinguish patients in different treatment response groups. Further integrative analysis of immune cell composition and enriched cancer-related pathways defined three immune microenvironment phenotypes: immune-enriched, tumor-proliferation, and fibroblast-enriched. Immune-enriched patients exhibited more significant tumor shrinkage compared to the other two types, and the response group had a higher proportion of immune-enriched patients.
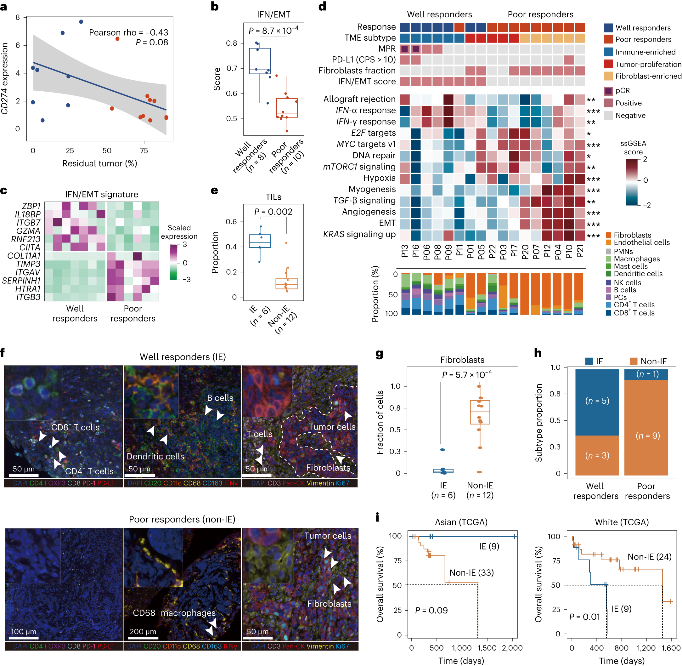
The analysis of non-responsive patients found that their tumors harbored higher densities of immunosuppressive cells such as M2 macrophages and fibroblasts. These patients may require a combination of immune-suppressing strategies to benefit from immunotherapy. Using multiple immunofluorescence staining techniques, the research team further characterized the spatial distribution of the microenvironment in 2 non-responsive patients, classified as immune-excluded and immune-suppressive. In immune-excluded tumors, immune cells accumulated at the tumor margin with limited infiltration into the core, while the immune-suppressive tumor core accumulated more pro-tumor macrophages and fewer B cells.
By analyzing the immune repertoire (TCR-seq) of tumor tissues and peripheral blood mononuclear cells (PBMCs), the researchers found that T cell receptor (TCR) diversity in PBMCs and the clonality of tumor-infiltrating T cells (TILs) were positively correlated with treatment response. This suggests that the degree of immune activation in peripheral blood and the infiltration of T cells into the tumor microenvironment may play important roles in predicting the efficacy of immunotherapy.
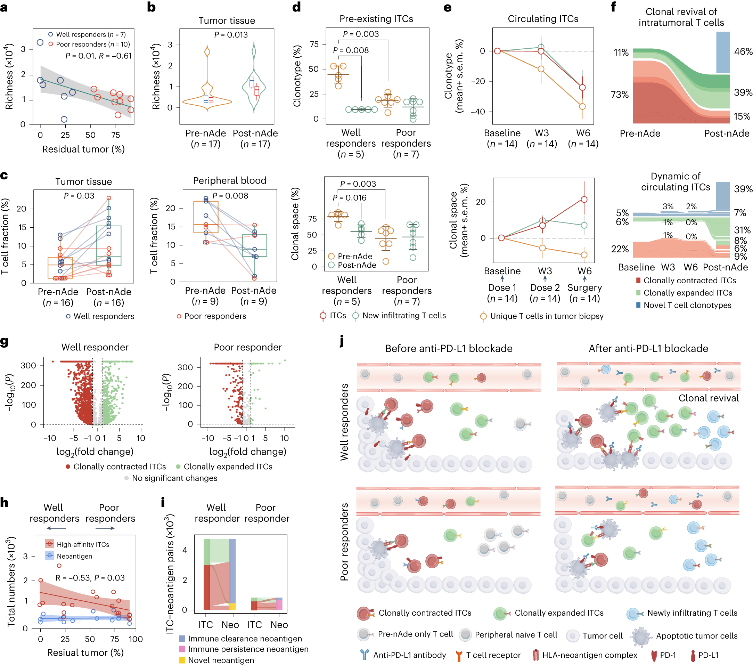
The study provides evidence of the safety and effectiveness of neoadjuvant mono-immunotherapy using Adebrelimab in treating locally advanced resectable esophageal squamous cell carcinoma. It also suggests new approaches for predicting the efficacy of tumor immunotherapy through multi-omics studies. The "IFN/EMT score," derived from transcriptome data, immune microenvironment phenotypes, and TCR repertoire analysis, may serve as a potential biomarker for predicting immunotherapy efficacy in patients with esophageal squamous cell carcinoma. These findings establish a foundation for optimizing and personalizing immunotherapy strategies for patients with this type of cancer.

Reference:
Yin, J., Yuan, J., Li, Y. et al. Neoadjuvant adebrelimab in locally advanced resectable esophageal squamous cell carcinoma: a phase 1b trial. Nat Med (2023).