IDeate-Lung01 Phase 2 Trial: Promising Response Rates with Ifinatamab Deruxtecan in Advanced Small Cell Lung Cancer
An interim analysis of the dose-optimization segment of the current IDeate-Lung01 phase 2 trial revealed that ifinatamab deruxtecan (I-DXd) remains to show promising objective response rates in patients with pretreated extensive-stage small cell lung cancer (ES-SCLC). These findings were highlighted today during a press conference and will be shared in an oral presentation (OA04.03) on Sunday at the 2024 World Conference on Lung Cancer (#WCLC24), organized by the International Association for the Study of Lung Cancer.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
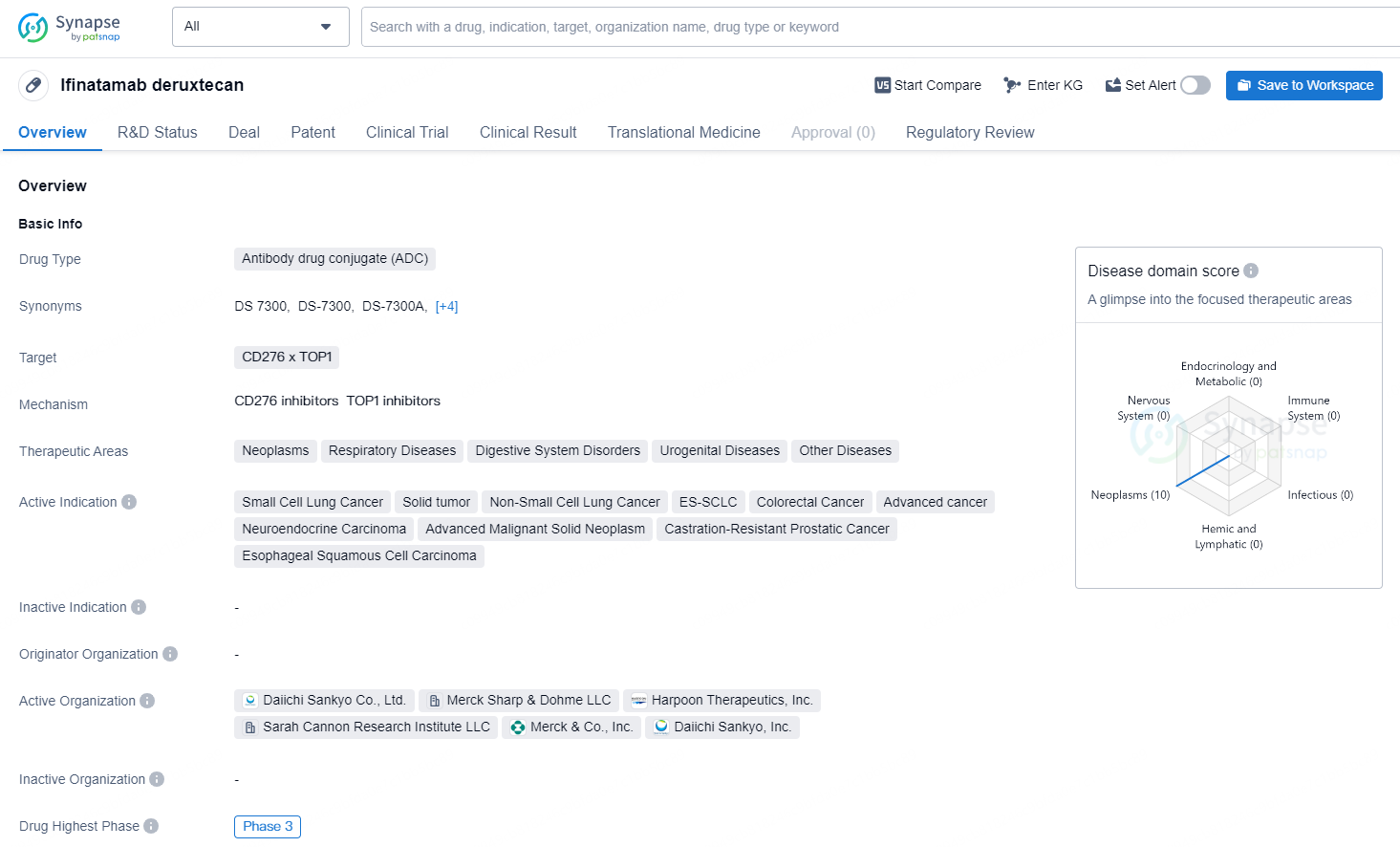
Ifinatamab deruxtecan is an antibody drug conjugate (ADC) specifically designed as a potential first-in-class treatment targeting B7-H3. This ADC was discovered by Daiichi Sankyo (TSE: 4568) and is under joint development by Daiichi Sankyo and Merck (NYSE: MRK), identified as MSD outside the United States and Canada.
Small cell lung cancer (SCLC) ranks as the second most prevalent form of lung cancer, accounting for around 15% of lung cancer cases. SCLC is characterized by its aggressive nature and rapid progression to a metastatic stage, where the five-year survival rate plummets to only 3%. Approximately 65% of SCLC tumors exhibit moderate to high levels of B7-H3 protein, a marker linked to disease advancement and poor prognosis.
"Many patients undergoing treatment for small cell lung cancer face swift disease progression, highlighting the significant unmet need in advanced-stage care," stated Charles M. Rudin, MD, PhD, Deputy Director of Memorial Sloan Kettering Cancer Center and Co-Director of the Fiona and Stanley Druckenmiller Center for Lung Cancer Research. "Initial data from the first segment of the IDeate-Lung01 trial indicate that ifinatamab deruxtecan might play a crucial role in managing patients with previously treated extensive-stage small cell lung cancer, meriting further investigation."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
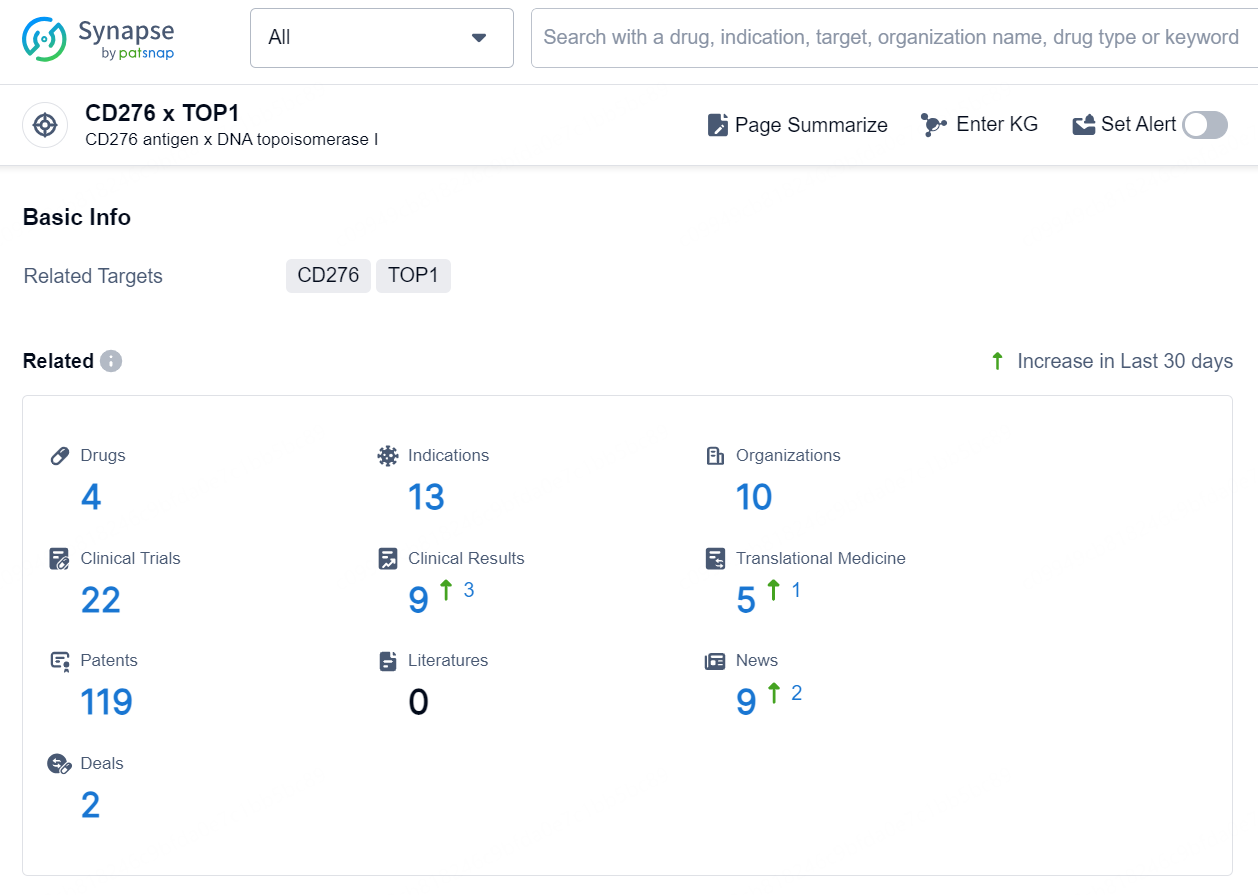
According to the data provided by the Synapse Database, As of September 11, 2024, there are 4 investigational drugs for the CD276 x TOP1 target, including 13 indications, 10 R&D institutions involved, with related clinical trials reaching 22, and as many as 119 patents.
Ifinatamab deruxtecan is an antibody drug conjugate (ADC) that targets CD276 and TOP1. It is being developed for the treatment of a wide range of therapeutic areas, including neoplasms, respiratory diseases, digestive system disorders, urogenital diseases, and other diseases. The drug is currently being investigated for the treatment of small cell lung cancer, solid tumor, non-small cell lung cancer, ES-SCLC, colorectal cancer, advanced cancer, neuroendocrine carcinoma, advanced malignant solid neoplasm, castration-resistant prostatic cancer, and esophageal squamous cell carcinoma.