IDRx shares initial findings from its ongoing Phase 1 StrateGIST trial at CTOS 2023, illustrating the promise of IDRX-42 for GIST patients
IDRx, Inc., a company working in clinical-stage biopharmaceuticals aimed at revolutionizing cancer treatment with specially designed precision therapies, reported preliminary clinical results from the dosage increase segment of its current Phase 1 StrateGIST trial of IDRX-42 in patients suffering from metastatic and/or inoperable gastrointestinal stromal tumors who have undergone at least one previous tyrosine kinase inhibitor therapy regime.
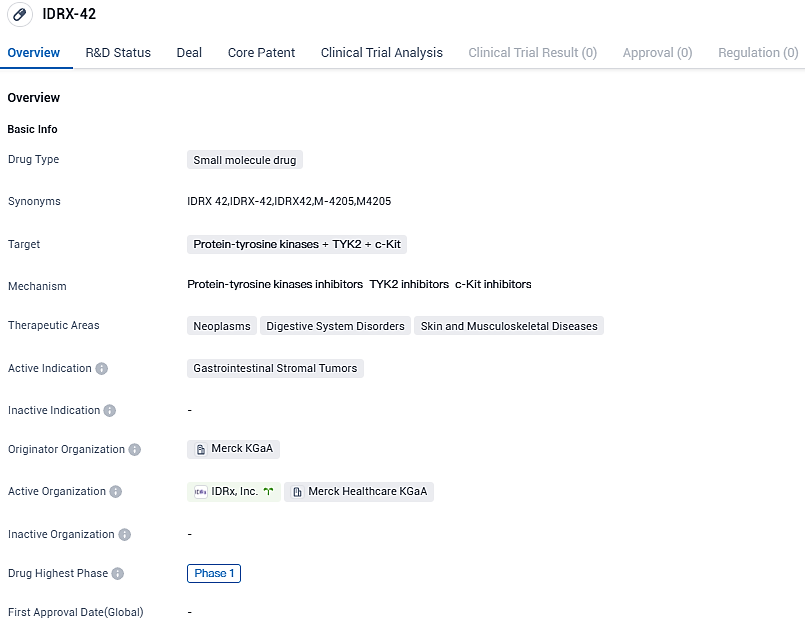
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
IDRX-42, as a highly efficient, multi-mutation-specific KIT inhibitor, targets all major categories of initial driver mutations and resistance mutations in patients with KIT-mutant GIST. "Promptly, there is a crucial requirement for novel therapies like IDRX-42.
It focusses on primary activating drivers and secondary resistance mutations in KIT after the failure of imatinib, which usually comes with a poor prognosis in advanced GIST," stated Prof. Schöffski. "Preliminary findings indicate promising and clinically significant antitumor activity in treating a challenging and highly refractory GIST patient group, thus advocating the exploration of IDRX-42 as an earlier therapy."
"We are greatly motivated by these preliminary results of IDRX-42, endorsing a possible superior profile to enhance the results for patients with GIST throughout all treatment stages. We believe this still remains one of the major unsolved medical requirements in targeted oncology," stated Ben Auspitz, co-founder and CEO of IDRx.
Pursuing further, Ben Auspitz stated, "Importantly, these initial findings will assist us in strategizing conversations with doctors about progress plans for IDRX-42 with GIST patients in the preliminary stages of treatment to avoid the onset of genomic-driven resistance mutations.
Suppressing such resistance mutations in KIT earlier on, rather than attempting to treat a clinically varied disease in any individual with several resistant clones, is likely to be more generally beneficial for GIST patients."
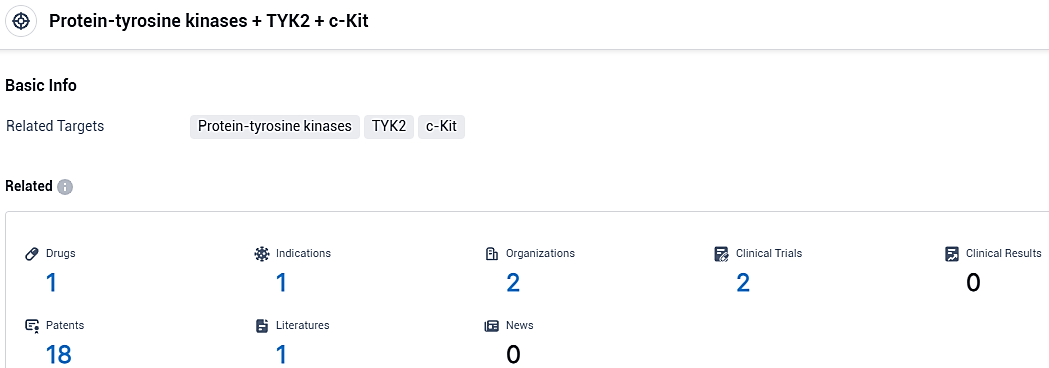
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 9, 2023, there are 1 investigational drugs for the Protein-tyrosine kinases and TYK2 and c-Kit target, including 1 indications, 2 R&D institutions involved, with related clinical trials reaching 2, and as many as 18 patents.
IDRX-42 is a potent, oral, highly selective KIT inhibitor targeting all major categories of activating and resistance mutations in patients with KIT-mutant GIST. In preclinical studies, IDRX-42 demonstrated superior antitumor activity compared to imatinib, the current first-line of therapy, in GIST human xenograft models. While IDRX-42 shows promise in the treatment of digestive system disorders and skin and musculoskeletal diseases, further research and clinical trials are needed to determine its efficacy and safety in these areas.