Research, Patent, and Literature Data behind the Early ADC Drug ORM-6151 Purchased by Bristol-Myers Squibb at a Huge Cost
Bristol Myers Squibb is acquiring an antibody-drug conjugate ORM-6151 from multinational biotechnology startup Orum Therapeutics to treat bone and blood cancers. Under the terms of the deal, Bristol Myers will pay Orum $100 million for the program and commit to paying as much as $80 million more if certain milestones are met.
ORM-6151 is a monoclonal antibody drug that targets CD33 and GSPT1, developed by Orum Therapeutics, Inc. It is primarily used in treating hemic and lymphatic diseases and neoplasms, including acute myeloid leukemia, high-risk myelodysplastic syndrome, and hematologic neoplasms. These diseases are related to the blood and lymphatic system, as well as various types of cancers. ORM-6151 has received approval from regulatory authorities for the IND approval phase, suggesting its potential as a treatment option for patients with these diseases.
Bristol Myers is one of several large drugmakers now investing ADCs in a major way. The commercial success of drugs such as AstraZeneca’s Enhertu and Seagen’s Adcetris has prompted a recent flurry of ADC-related dealmaking, including one last month between Merck and Co. and Daiichi Sankyo.
ORM-6151 Overview
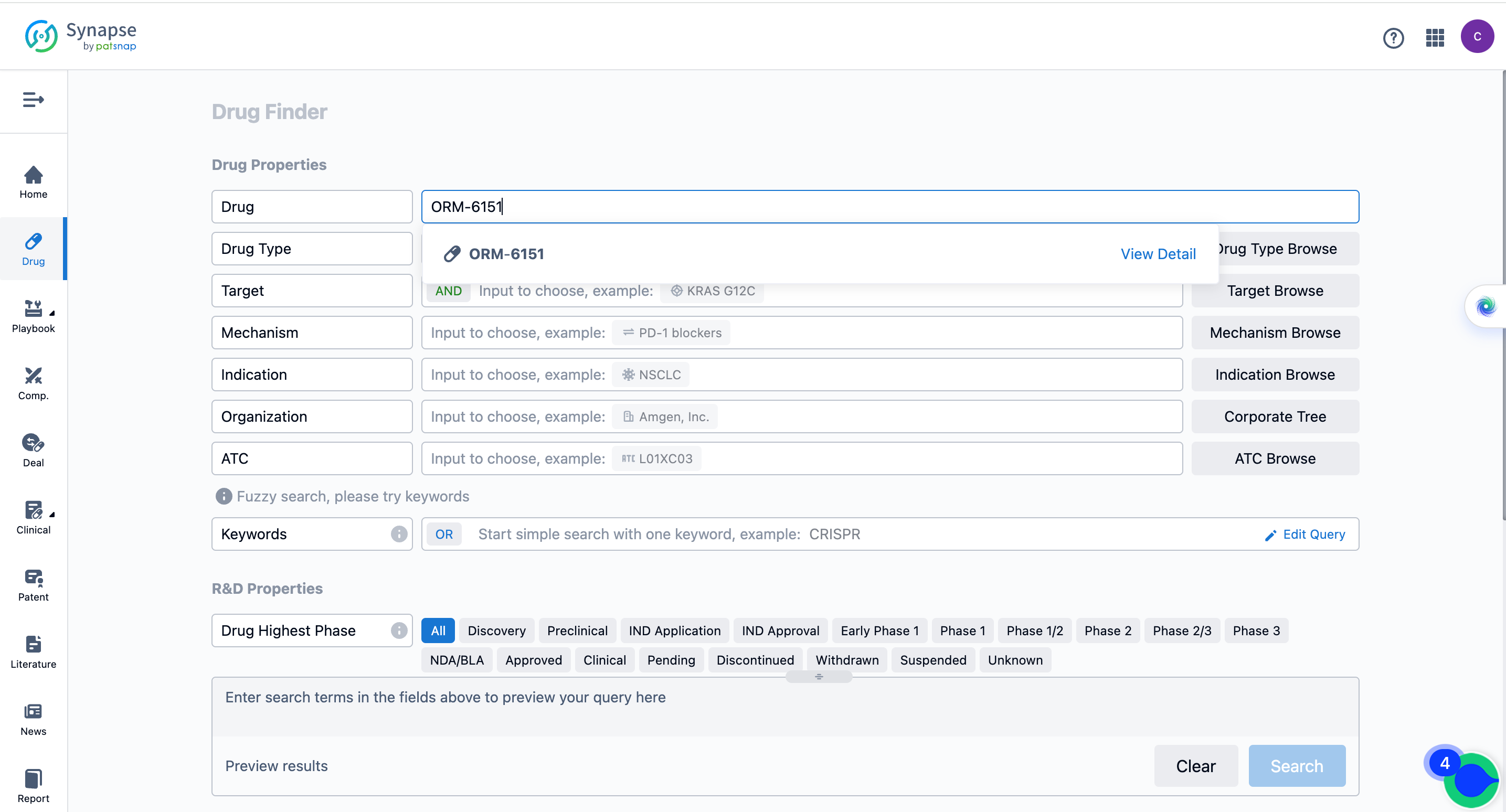
In the Patsnap Synapse Database, one can query the basic information, R&D Status, Deal, Core Patent, Clinical trials, and Clinical results, etc. of the drug ORM-6151 with a single click. Detailed data can be seen in the figure below.
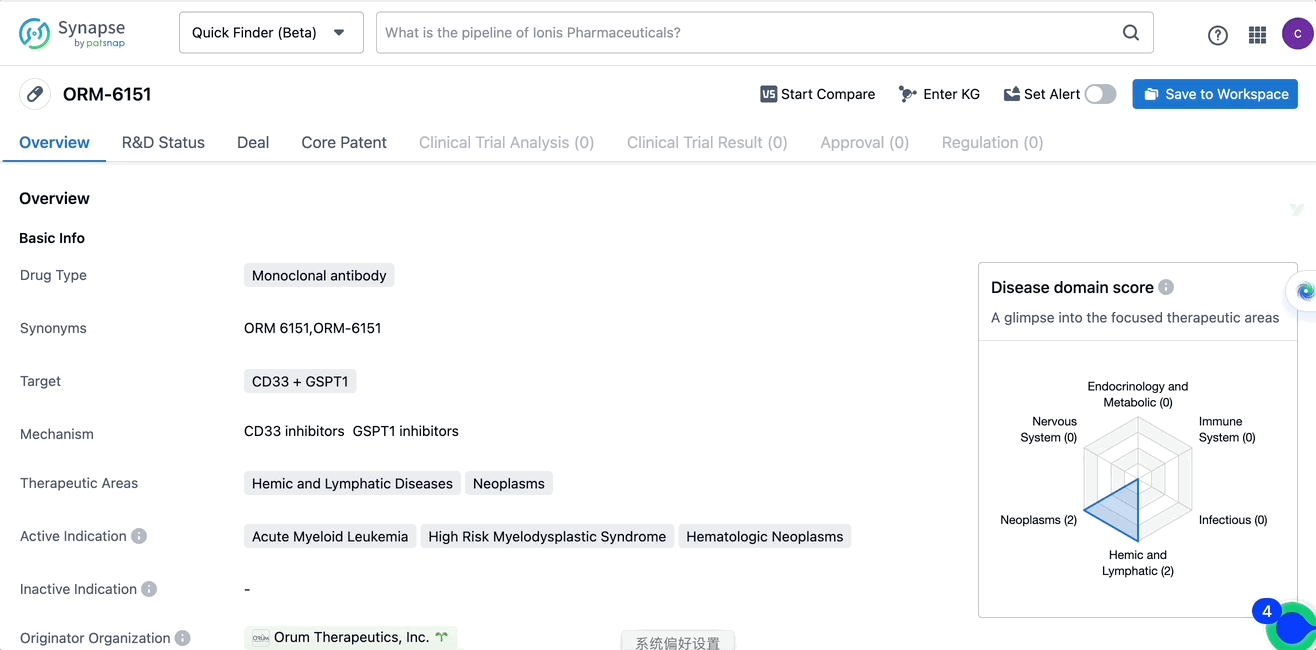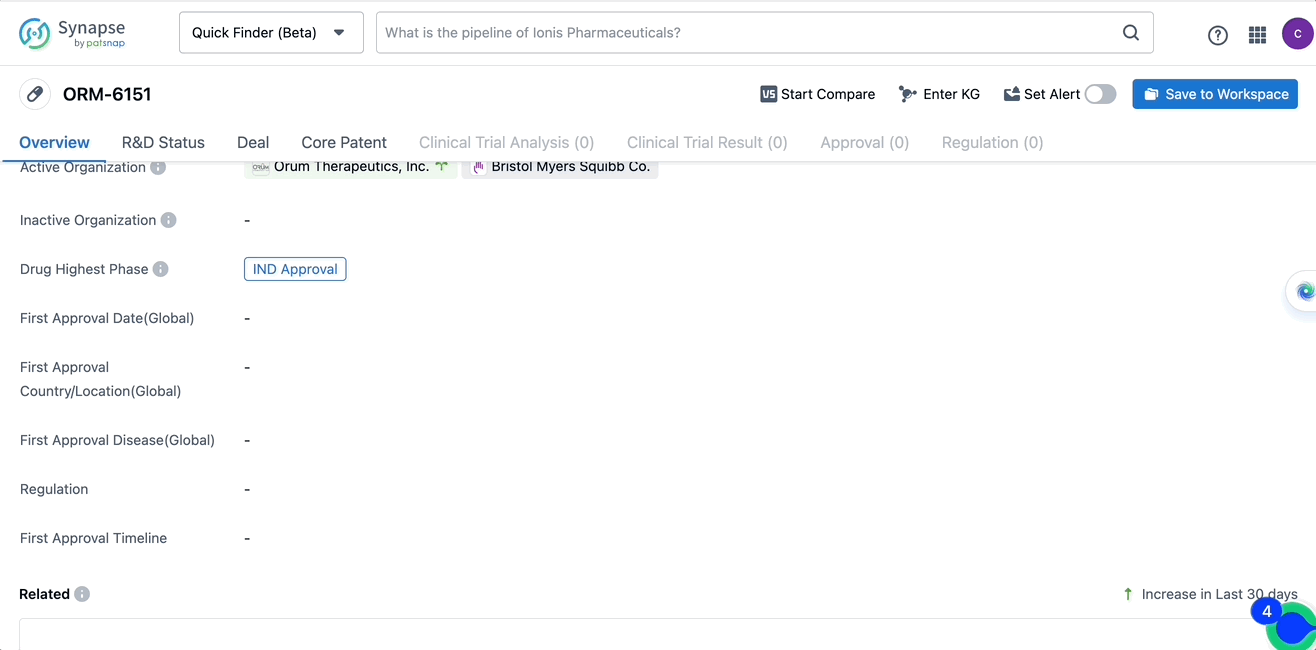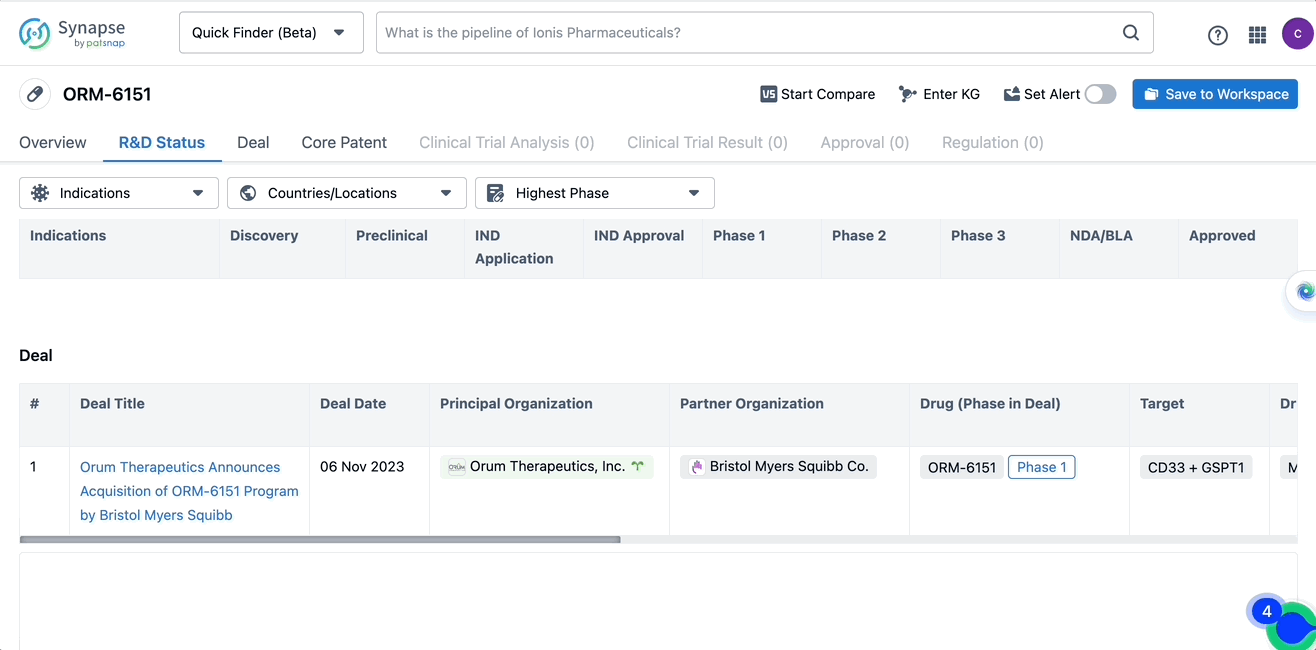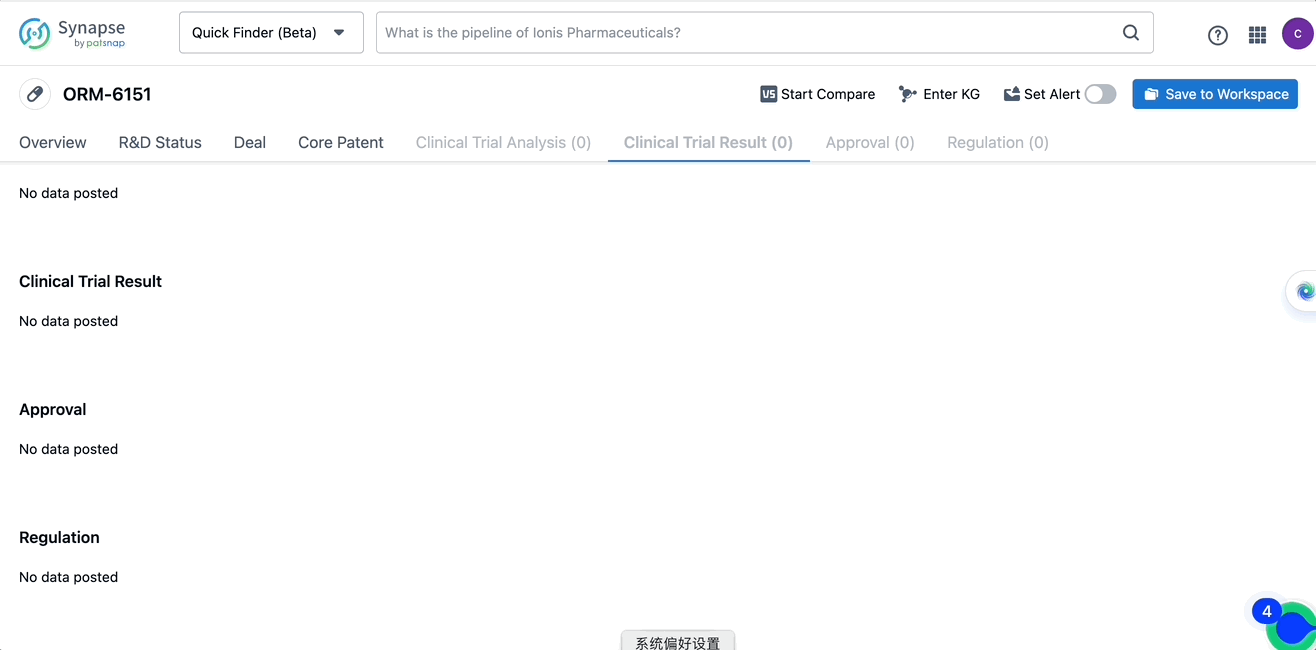
Source, Patsnap Synapse
ORM-6151 Biological Sequence
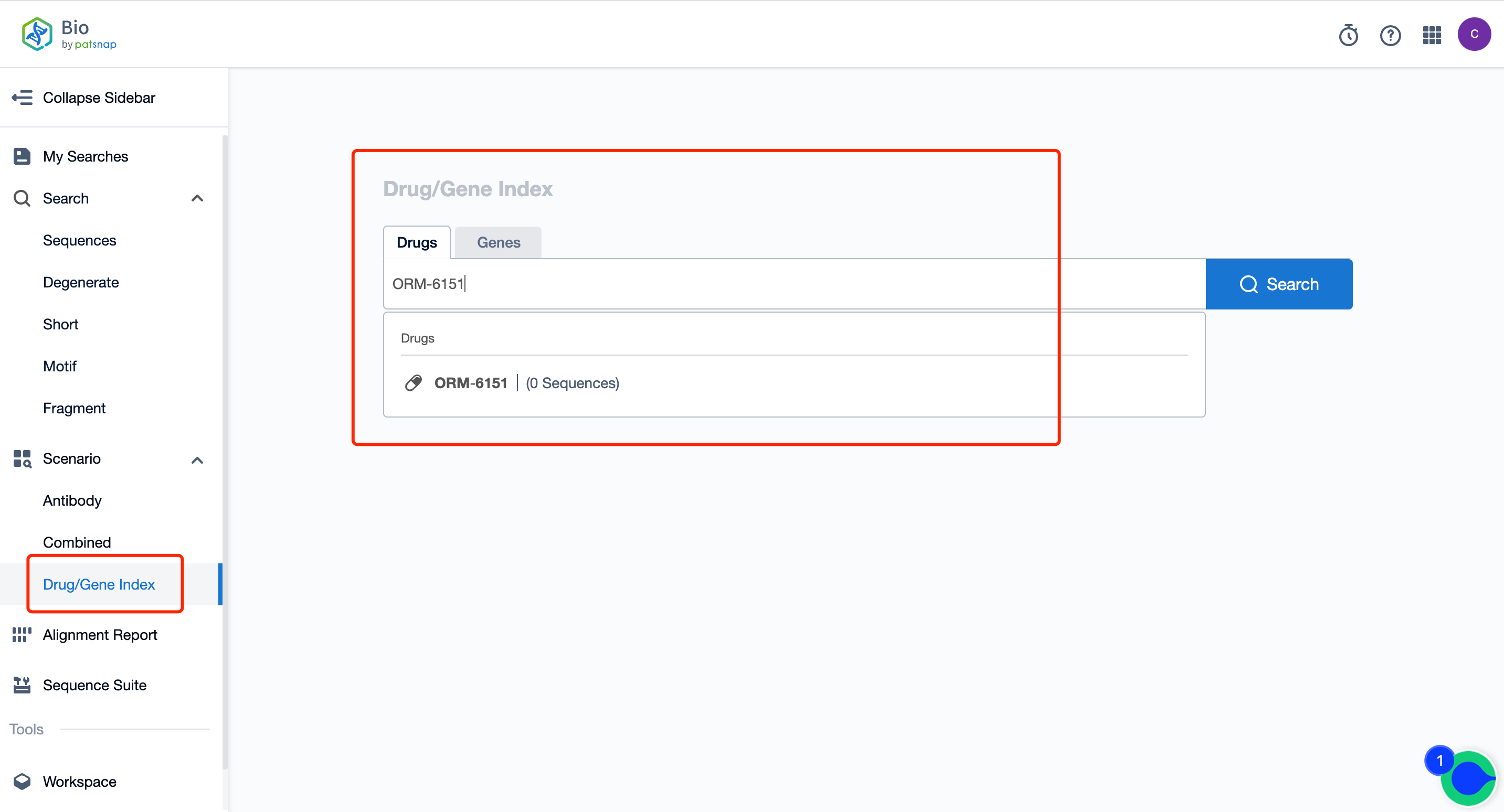
Create a free account in the Patsnap Bio Sequence Database. Enter the homepage and input the drug name ORM-6151 directly in the "Drug/Gene Index". Retrieve its sequence with one click. As shown in the figure below, the global ORM-6151 sequence data has not yet been published.
Source, Patsnap Bio
ORM-6151 Patent
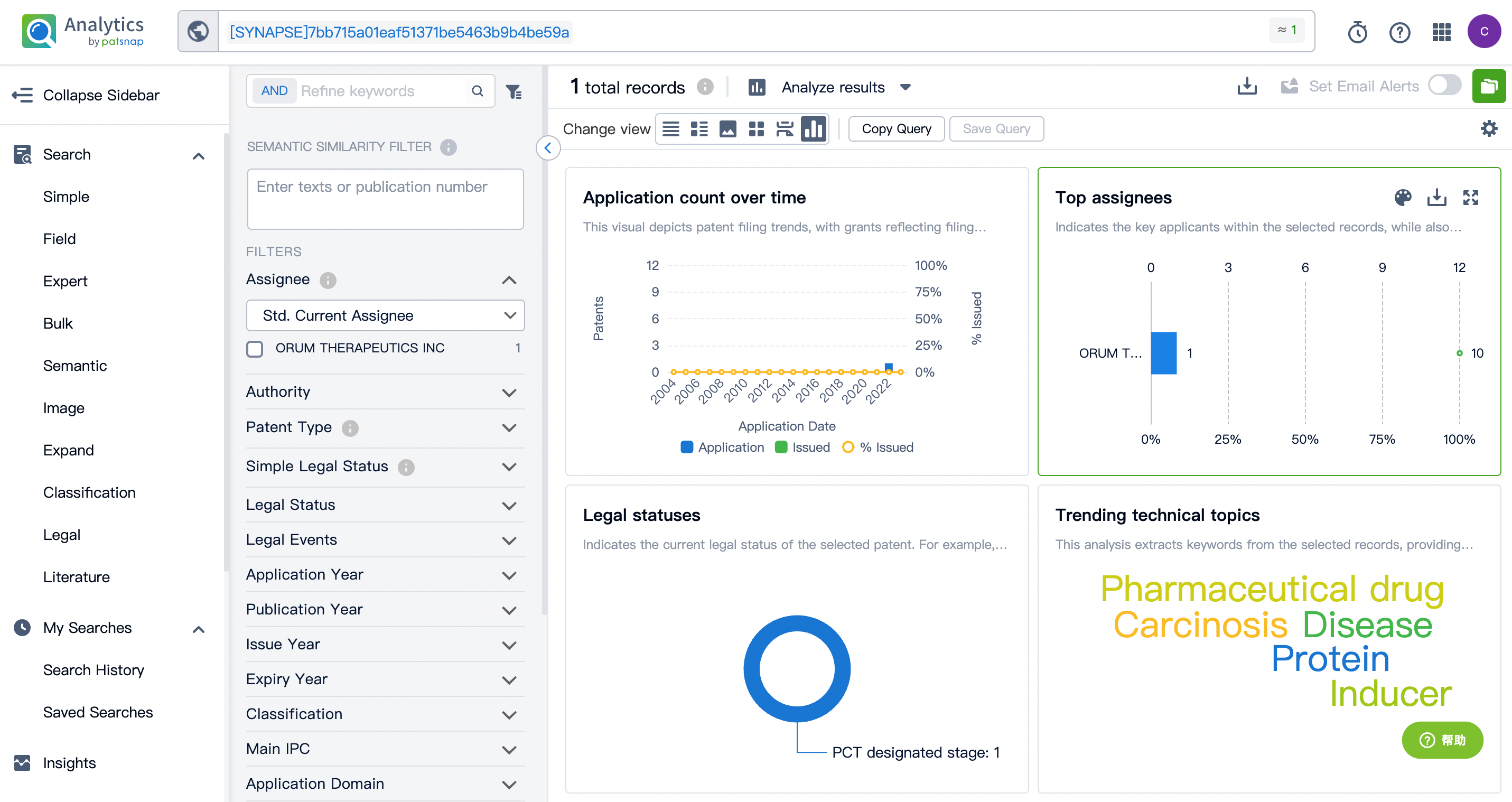
A patent for the drug ORM-6151 was found in the patent database. This patent, labeled WO2023037268A1, was applied for by Orum Therapeutics in 2022. The trending technology topics outlined in this patent include pharmaceutical drugs, Carcinosis, diseases, proteins, inducers, antibody-drug conjugates, and inducers. Please refer to the following figure for detailed patent information.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.