Immatics Releases Phase 1b Results for ACTengine® IMA203 TCR-T Targeting PRAME in Melanoma
Immatics N.V. (NASDAQ: IMTX, referred to as "Immatics" or the "Company"), a biopharmaceutical firm in the clinical stage that focuses on discovering and developing T cell-redirecting therapies for cancer immunotherapy, has released new Phase 1b clinical results regarding ACTengine® IMA203 TCR-T aimed at targeting PRAME in patients with melanoma. Additionally, the Company has provided an update on SUPRAME, the forthcoming Phase 3 trial designed to assess the effectiveness of IMA203 in individuals with metastatic melanoma.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The results from the ongoing Phase 1b study will be shared by Martin Wermke, M.D., on Friday, October 11, 2024, during the first plenary session focused on Developmental Immunotherapy (Cellular Immunotherapy, Vaccines, & New Checkpoints) at the Society for Melanoma Research Congress 2024. The slides presenting the IMA203 data can be found in the 'Events & Presentations' section of the Company's Investor & Media webpage. The conference will also include additional patient case presentations.
“Notable reductions in tumor size and lasting responses, alongside significant progression-free survival and overall survival rates following a single administration of ACTengine® IMA203 in this cohort of patients who have undergone multiple lines of systemic therapies, highlight the potential benefits of IMA203 for individuals with metastatic melanoma,” stated Martin Wermke, M.D., the Coordinating Investigator for the ACTengine® IMA203 TCR-T trial. “These findings reinforce the therapeutic promise of IMA203 and provide a compelling case for the accelerated late-stage clinical development of this candidate.”
“We are excited by the clinical findings, which reinforce our belief in the durability and long-term effectiveness of ACTengine® IMA203, as evidenced by the positive median progression-free survival observed in patients from the dose expansion group. Notably, 12 out of 26 patients experienced over a 50% reduction in tumor lesions with a median PFS of 13.4 months,” mentioned Cedrik Britten, M.D., Chief Medical Officer at Immatics. “We are confident that showcasing this dataset, coupled with our recent engagement with the FDA—resulting in a pivotal trial framework with progression-free survival designated as the primary endpoint for full approval—positions us well to further advance IMA203 in the treatment of metastatic melanoma after the second line.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
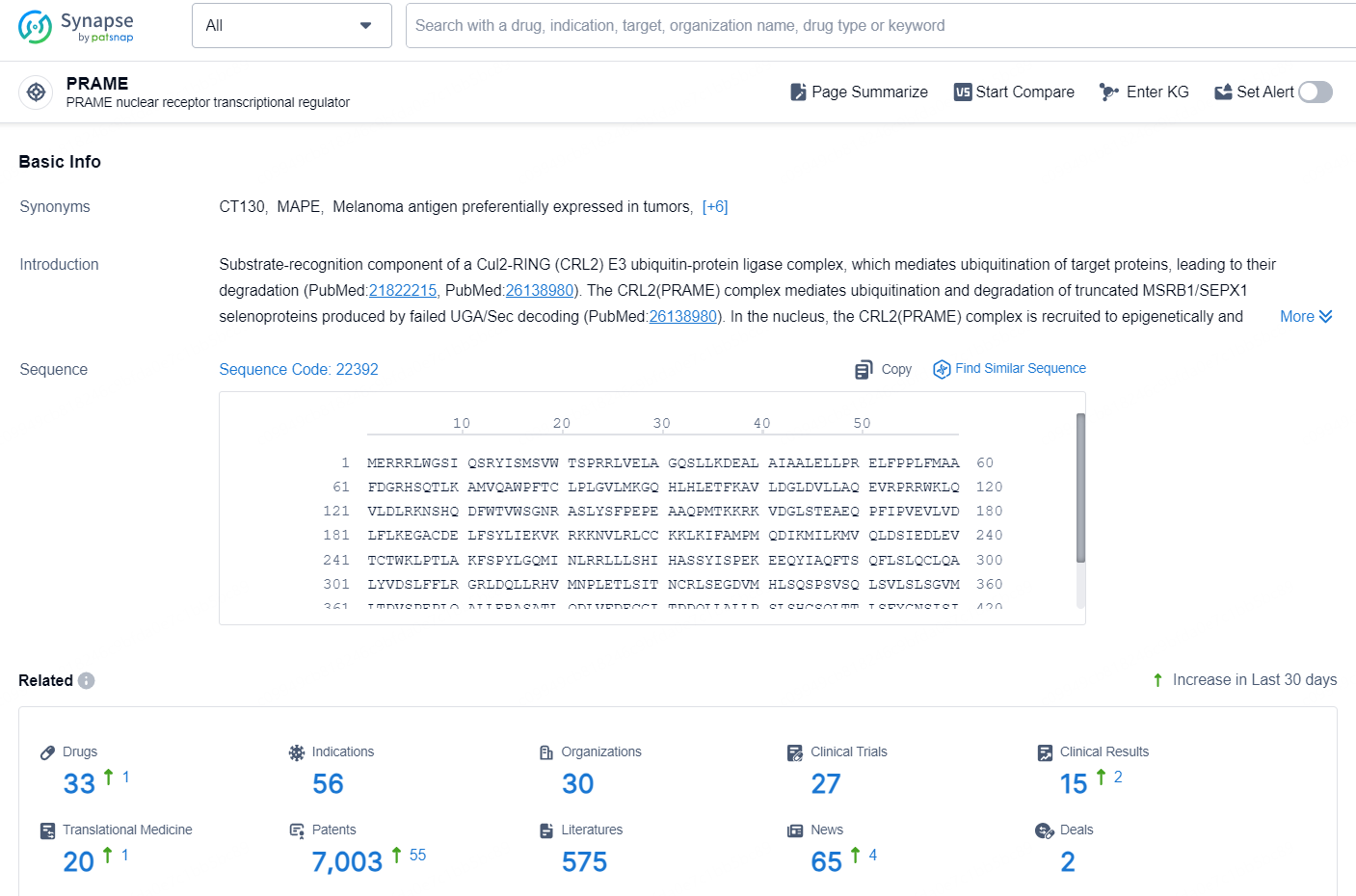
According to the data provided by the Synapse Database, As of October 10, 2024, there are 33 investigational drugs for the PRAME targets, including 56 indications, 30 R&D institutions involved, with related clinical trials reaching 27, and as many as 7003 patents.
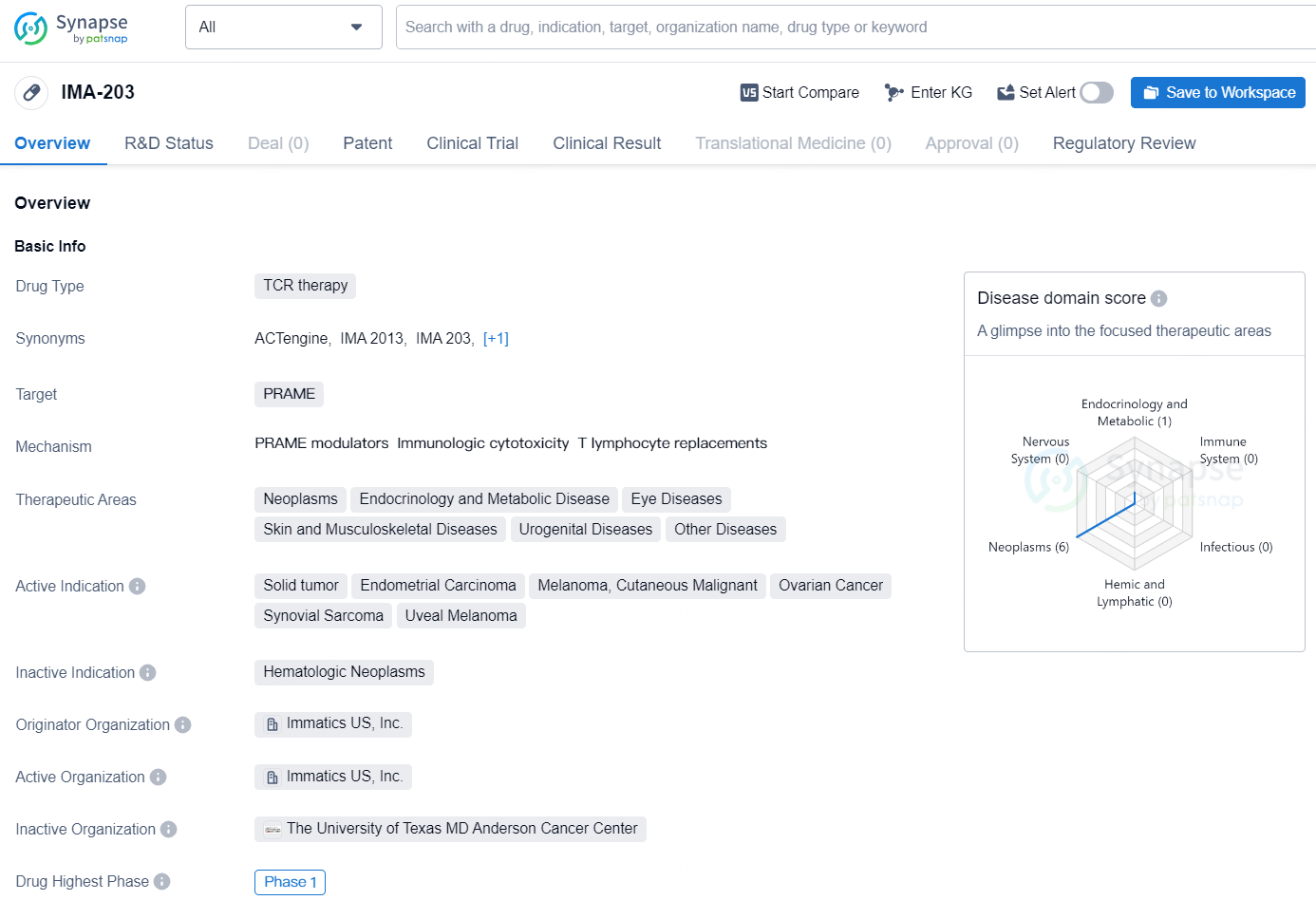
The drug IMA-203 is a TCR (T-cell receptor) therapy that targets PRAME and is being developed by Immatics US, Inc. The therapeutic areas for IMA-203 include neoplasms, endocrinology and metabolic diseases, eye diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases. The active indications for the drug are solid tumor, endometrial carcinoma, melanoma, cutaneous malignant, ovarian cancer, synovial sarcoma, and uveal melanoma.