TREMFYA® (Guselkumab) Demonstrates Significant Efficacy in Crohn's Disease and Ulcerative Colitis Patients
Johnson & Johnson (NYSE: JNJ) has revealed data regarding TREMFYA® (guselkumab) related to both Crohn’s disease (CD) and ulcerative colitis (UC), demonstrating significant rates of endoscopic remission in patients who are either naive to biologics or have not responded to previous biologic treatment (including UC patients resistant to JAK inhibitors), signifying a return to normal intestinal mucosa appearance. These subgroup analyses are derived from combined data from the Phase 3 GALAXI 2 & 3 trials involving TREMFYA® for adults suffering from moderately to severely active CD, as well as the Phase 3 QUASAR maintenance trial for adults with moderately to severely active UC. This information is part of 19 abstracts from Johnson & Johnson that will be presented at the United European Gastroenterology (UEG) Week 2024. TREMFYA® is currently undergoing evaluation by the European Medicines Agency (EMA) for its use in adults with moderately to severely active UC and CD.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“The findings indicate that TREMFYA has the potential to provide a distinct treatment alternative for individuals with CD and UC, encompassing those initiating biologic therapy for the first time, as well as those who have not responded to previous biologics and are traditionally less likely to benefit from other treatments,” remarked Esi Lamousé-Smith, M.D., Ph.D., who serves as Vice President and Gastroenterology Disease Area Lead at Immunology, Johnson & Johnson Innovative Medicine. “TREMFYA builds upon nearly thirty years of our expertise in IBD treatment and targeted innovation in the IL-23 pathway to meet the needs of individuals with ulcerative colitis and to offer significant improvements in symptoms and the possibility of sustained remission.”
Endoscopic remission in patients naive to biologics
In the combined Phase 3 GALAXI 2 & 3 study data, TREMFYA® exhibited higher rates of endoscopic remission compared to ustekinumab at Week 48 in patients with CD who had never received biologic treatment. Specifically, endoscopic remission was seen in 44% of individuals administered TREMFYA® 100 mg every eight weeks (q8w) via subcutaneous (SC) injection and in 46.1% of those receiving TREMFYA® 200 mg every four weeks (q4w) SC injection, contrasting with 29.8% for ustekinumab-treated patients.
In the Phase 3 QUASAR trial, TREMFYA® also demonstrated superior endoscopic remission rates compared to placebo at Week 44 among biologic and JAK inhibitor-naive patients with UC. Endoscopic remission occurred in 38.1% of subjects given TREMFYA® 100 mg q8w SC injection and in 41.7% of those receiving TREMFYA® 200 mg q4w SC injection, while only 20.4% of patients on placebo achieved endoscopic remission.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
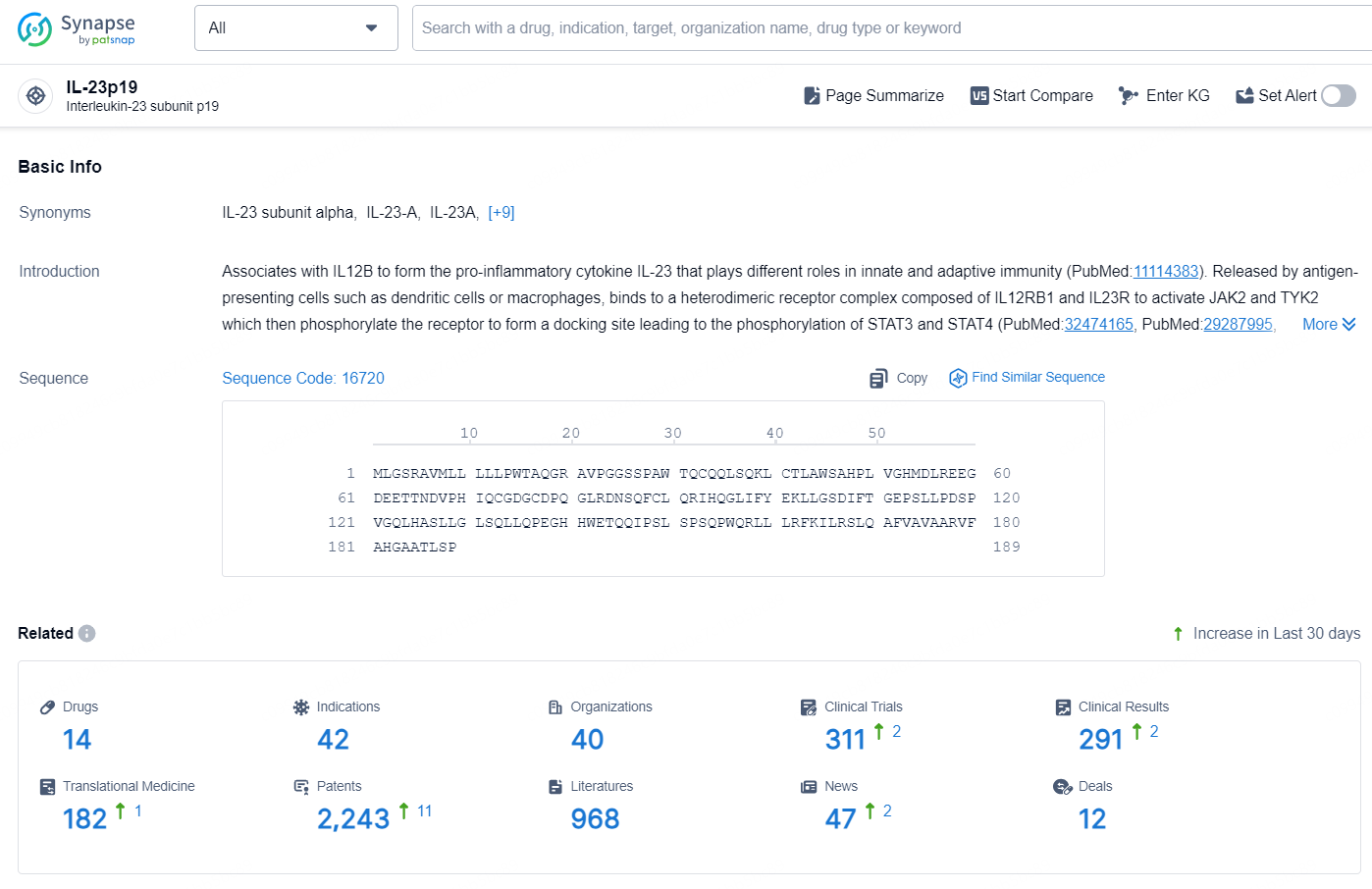
According to the data provided by the Synapse Database, As of October 10, 2024, there are 14 investigational drugs for the IL-23p19 targets, including 42 indications, 40 R&D institutions involved, with related clinical trials reaching 311, and as many as 2243 patents.
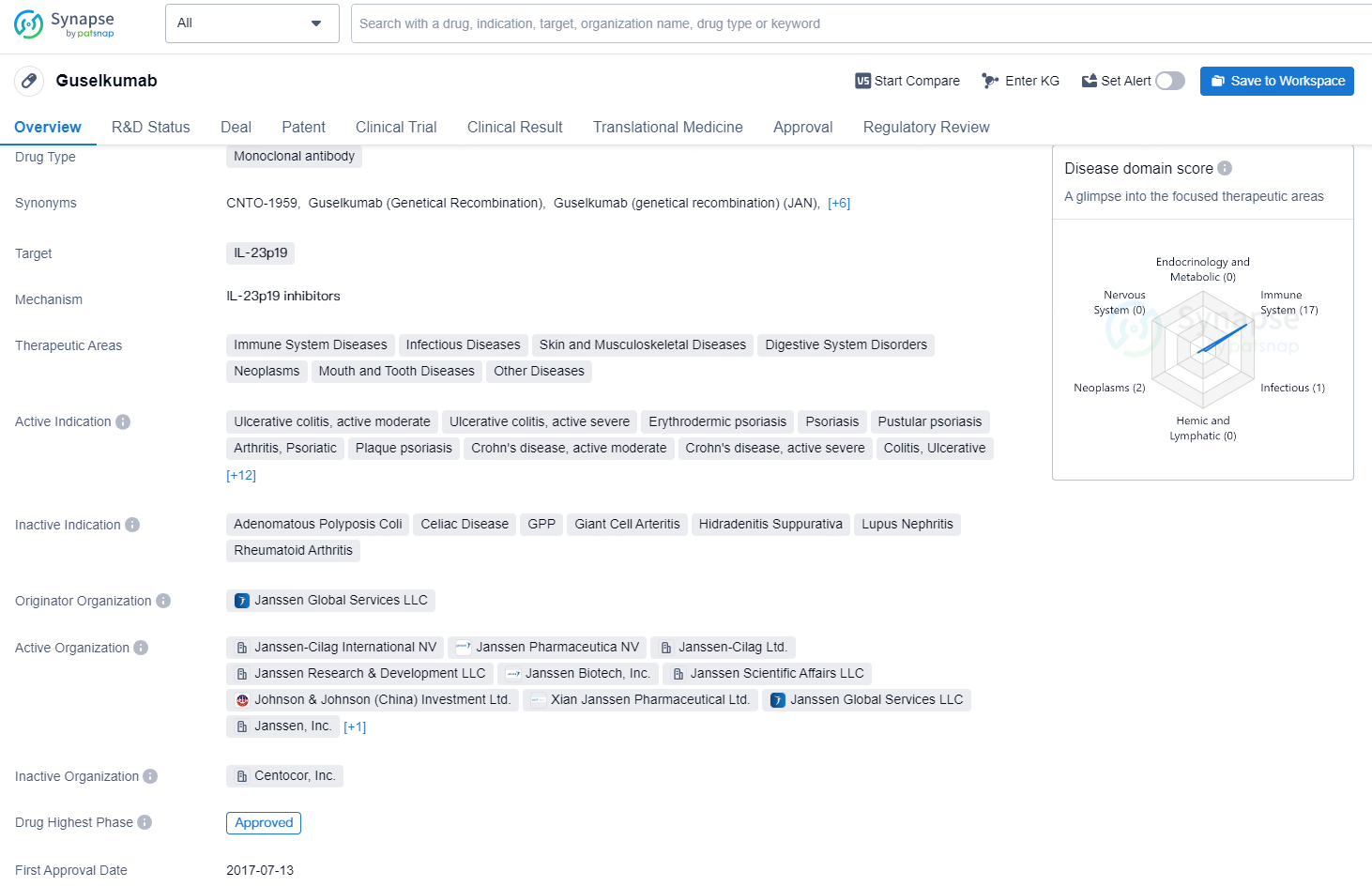
The drug Guselkumab is a monoclonal antibody that targets IL-23p19. It is mainly used in the treatment of a wide range of conditions including immune system diseases, infectious diseases, skin and musculoskeletal diseases, digestive system disorders, neoplasms, mouth and tooth diseases, and other conditions.