Pfizer's TALZENNA® combined with XTANDI® extends overall survival in the Phase 3 TALAPRO-2 study
Pfizer Inc. (NYSE: PFE) has revealed encouraging topline findings from the final pre-specified overall survival (OS) assessment of the TALAPRO-2 trial involving TALZENNA® (talazoparib), an oral poly ADP-ribose polymerase (PARP) inhibitor, in conjunction with XTANDI® (enzalutamide), an androgen receptor pathway inhibitor (ARPI), for patients diagnosed with metastatic castration-resistant prostate cancer (mCRPC). The findings indicated a statistically significant and clinically relevant enhancement in final OS for both the overall patient population (cohort 1) and those with homologous recombination repair (HRR) gene mutations (cohort 2), when compared to treatment with XTANDI alone.
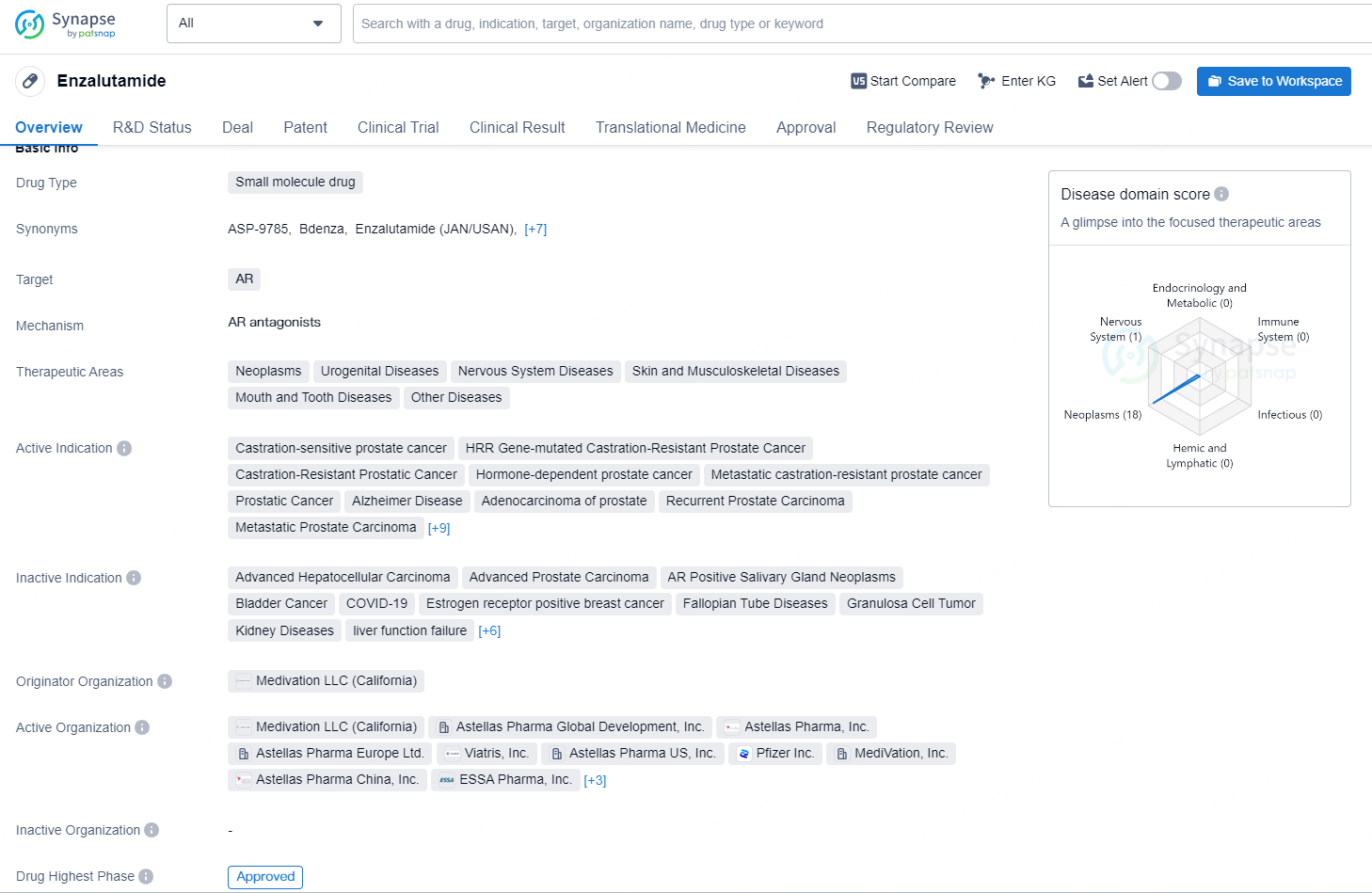
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The findings from TALAPRO-2 demonstrated that the combination of TALZENNA and XTANDI constitutes the first and sole PARP inhibitor alongside an ARPI that significantly enhances survival rates in patients suffering from metastatic castration-resistant prostate cancer, irrespective of their mutation status," stated Roger Dansey, M.D., Chief Development Officer for Oncology at Pfizer. "Our commitment at Pfizer is to propel scientific advancements in genitourinary tumors, and these promising TALAPRO-2 outcomes underscore our enduring dedication to raising survival rates for men with prostate cancer."
"The overall survival data suggest a potentially transformative effect of TALZENNA in conjunction with XTANDI for men diagnosed with metastatic castration-resistant prostate cancer," remarked Neeraj Agarwal, M.D., FASCO, a Professor and the Presidential Endowed Chair of Cancer Research at the Huntsman Cancer Institute, University of Utah, who serves as the global lead investigator for TALAPRO-2. "This stage represents the most severe and aggressive form of the disease, and the results from TALAPRO-2 bring critical hope to patients who are facing a significant lack of effective treatment options."
During the final analysis, a clinically significant enhancement in radiographic progression-free survival (rPFS) was observed in both groups compared to findings reported in The Lancet from an earlier primary analysis. Furthermore, the safety profile for the combination of TALZENNA and XTANDI was largely in line with the established safety records of each drug. Comprehensive results from TALAPRO-2 will be prepared for presentation at an upcoming medical congress, and this information will be shared with global health authorities to potentially facilitate regulatory submissions aimed at updating and possibly broadening the approved indications for TALZENNA.
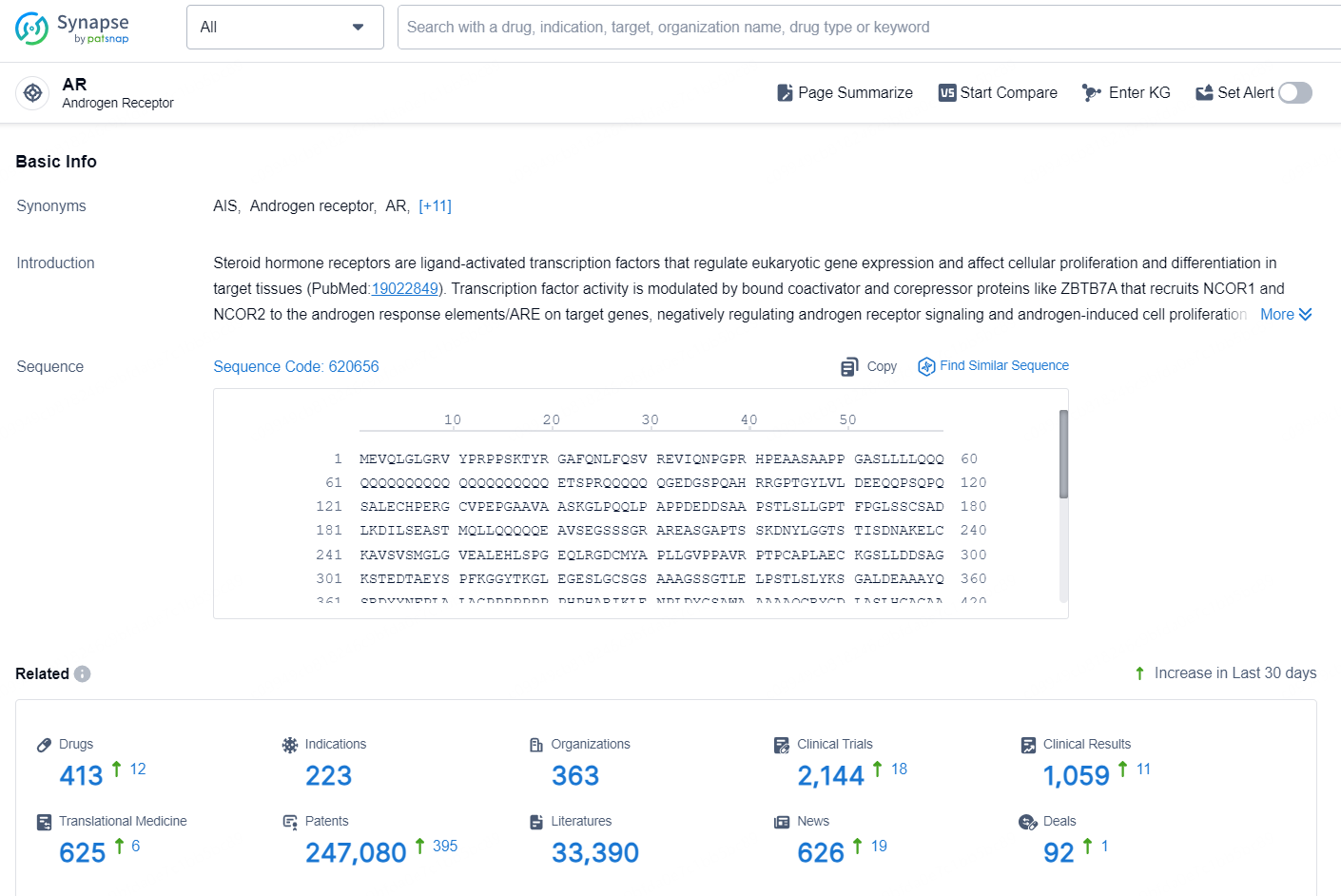
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 10, 2024, there are 413 investigational drug for the AR targets, including 223 indications, 363 R&D institutions involved, with related clinical trials reaching 2144, and as many as 247080 patents.
Enzalutamide is a small molecule drug that primarily targets the androgen receptor (AR). It is indicated for the treatment of a wide range of diseases, including neoplasms, urogenital diseases, nervous system diseases, skin and musculoskeletal diseases, mouth and tooth diseases, and other related ailments. Enzalutamide has been approved for various indications, including castration-sensitive prostate cancer, HRR gene-mutated castration-resistant prostate cancer, hormone-dependent prostate cancer, metastatic castration-resistant prostate cancer, and Alzheimer's Disease, among others.