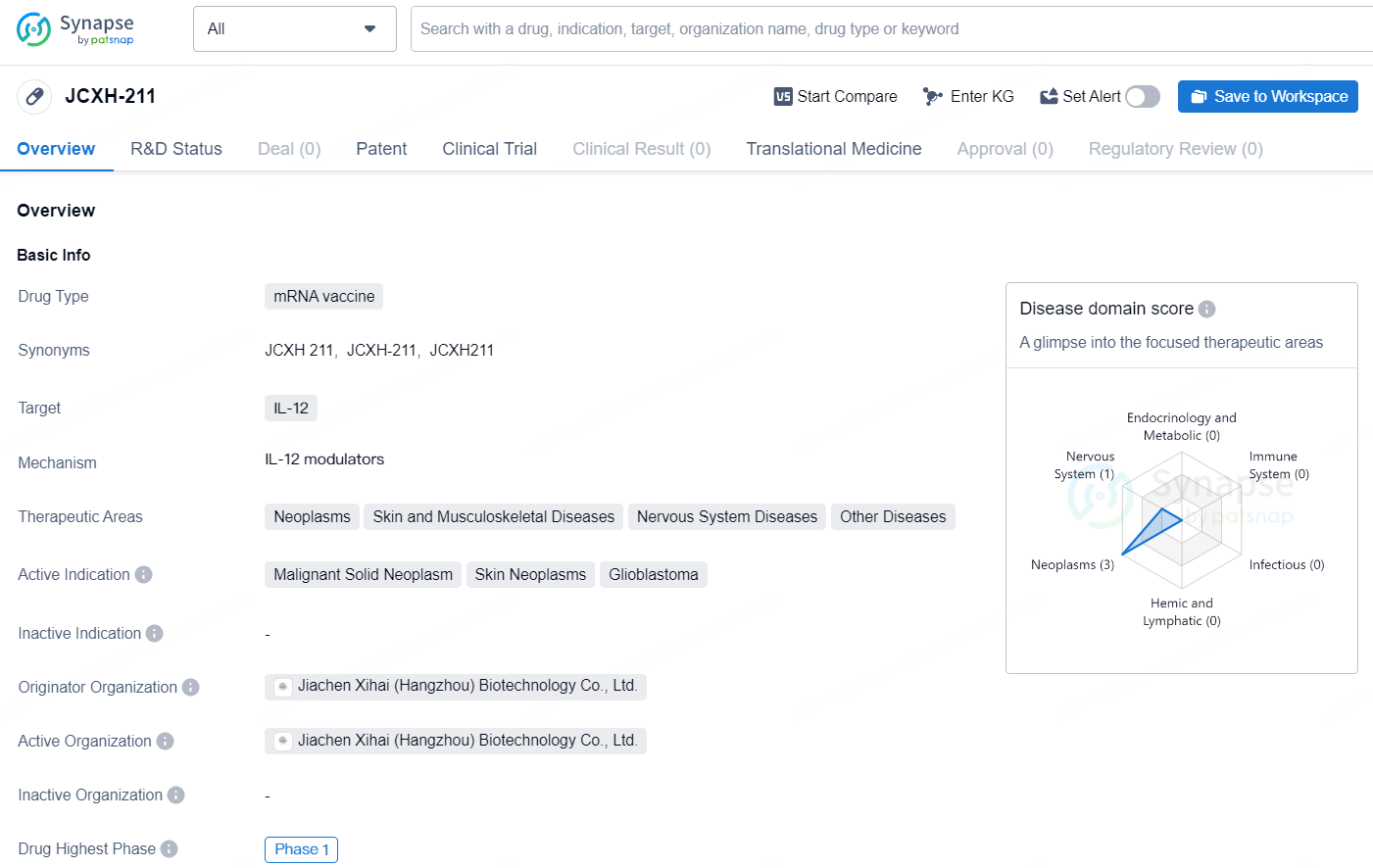
Immorna Biotherapeutics' JCXH-211 Receives FDA IND Approval for Phase 1/2 Solid Tumor Trial
Immorna Biotherapeutics, Inc., a clinical-stage biotech firm focused on the development of both self-replicating and traditional mRNA-based therapies and vaccines, revealed that the U.S. Food and Drug Administration has approved its investigational new drug application for JCXH-211 intravenous. This innovative, first-of-its-kind self-replicating mRNA encodes the modified human interleukin (IL)-12 protein.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The IND clearance permits Immorna to commence a Phase 1/2, multi-center, open-label, dose escalation and expansion trial of JCXH-211, which will be administered intravenously to patients with malignant solid tumors. The objectives of the trial are to evaluate safety and tolerance, establish the recommended Phase 2 dose (RP2D) for JCXH-211 IV when combined with a checkpoint inhibitor, and preliminarily assess the efficacy of the combination at the RP2D.
“Grounded on data from our preclinical studies and the clinical data from our JCXH-211 intratumoral administration trial, along with the candidate drug’s mechanism of action, we are optimistic that JCXH-211 IV, in combination with a checkpoint inhibitor, has the potential to synergistically enhance anti-tumor effects. We eagerly anticipate collaborating with investigators and patients to deliver this potentially groundbreaking therapy to those in urgent need of new and effective treatments,” stated NgocDiep Le, M.D., Ph.D., President and Global Chief Medical Officer of Immorna.
JCXH-211 is a pioneering lipid nanoparticle encapsulated srRNA, developed using Immorna’s proprietary technology, that encodes the engineered human IL-12 protein. In various preclinical animal and patient-derived xenograft models, the RNA replicon-induced anti-viral innate response combined with the IL-12 stimulated potent anti-cancer immunity endowed JCXH-211 with superior tumor-eradicating capabilities, surpassing similar preclinical candidates using conventional mRNA.
IL-12 is a naturally occurring cytokine integral to the body’s immune response against cancer. Despite demonstrating strong antitumor activity in preclinical trials, recombinant IL-12 protein treatments at tolerable doses in humans have not yielded clinical benefits. The primary obstacle for IL-12 protein treatments is the non-overlapping tolerability and therapeutic windows. Recombinant IL-12 is unstable in vivo and possesses a very short half-life, with frequent intravenous administration posing significant toxicity challenges.
Due to the intrinsic features of our srRNA technology, JCXH-211 IV enables prolonged expression of IL-12 primarily in tumor tissues, rather than normal tissues, thus modulating the tumor microenvironment and activating antitumor immune responses while minimizing systemic toxicity. JCXH-211 IV demonstrated an excellent safety profile in nonclinical studies conducted on rodents and non-human primates. Should it succeed, JCXH-211 IV could become a lifesaving and easily accessible treatment for cancer patients who have not responded to or are resistant to existing therapies.
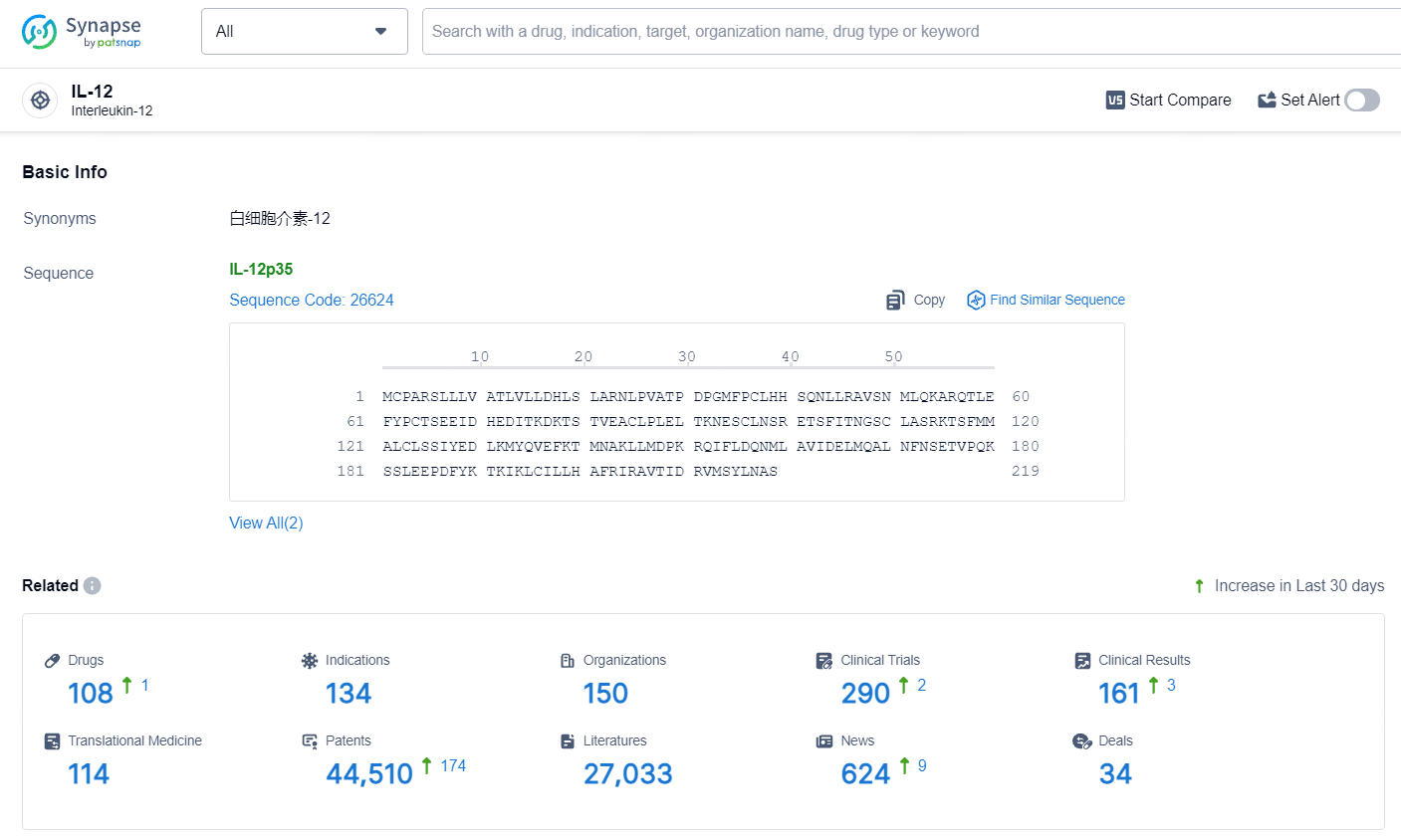
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 10, 2024, there are 108 investigational drugs for the IL-12 target, including 134 indications, 150 R&D institutions involved, with related clinical trials reaching 290, and as many as 44510 patents.
JCXH-211 represents an innovative mRNA vaccine targeting IL-12 with potential applications in the treatment of neoplasms, skin and musculoskeletal diseases, nervous system diseases, and other conditions. The drug is currently in Phase 1 of clinical development, and further research and clinical trials will be needed to determine its safety and efficacy in treating the indicated conditions.