Merck's KEYTRUDA Achieves Main Goal in Trial for Head and Neck Cancer
Merck (NYSE: MRK), referred to as MSD in regions outside the United States and Canada, announced that the Phase 3 KEYNOTE-689 trial assessing KEYTRUDA® (pembrolizumab), Merck’s PD-1 inhibitor, as a perioperative treatment for patients with newly diagnosed, resected, locally advanced head and neck squamous cell carcinoma (LA-HNSCC) at stages III or IVA successfully achieved its main objective of event-free survival (EFS).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The investigation focused on KEYTRUDA as a neoadjuvant intervention, which was subsequently followed by its use in conjunction with standard radiotherapy (with or without cisplatin) as adjuvant care after surgery, and later as maintenance therapy. This was compared to the use of adjuvant radiotherapy (with or without cisplatin) alone. An independent Data Monitoring Committee carried out a predefined first interim analysis revealing a statistically significant and clinically relevant enhancement in event-free survival (EFS) for those patients undergoing the KEYTRUDA perioperative regimen. Additionally, a significant statistical improvement in major pathological response (mPR), a crucial secondary endpoint, was noted in the KEYTRUDA group versus those receiving only adjuvant radiotherapy. The safety profile of KEYTRUDA aligned with outcomes from earlier studies, with no new safety concerns reported.
“These findings are groundbreaking, as KEYNOTE-689 represents the first positive trial in 20 years for patients with operable, locally advanced head and neck squamous cell carcinoma,” stated Dr. Marjorie Green, senior vice president and head of oncology, global clinical development at Merck Research Laboratories. “The statistically significant and clinically impactful results may significantly alter clinical practice and further emphasize the potential of KEYTRUDA for patients with earlier disease stages.”
A noticeable trend suggesting improved overall survival (OS) was observed among those receiving neoadjuvant KEYTRUDA, as well as in patients treated post-surgery with KEYTRUDA alongside standard radiotherapy (with or without cisplatin), followed by maintenance therapy with KEYTRUDA. However, the OS results did not achieve statistical significance for patients whose tumors had a PD-L1 Combined Positive Score (CPS) of ≥10 during this initial interim analysis. Due to the statistical testing hierarchy, formal analysis was not conducted for those with CPS ≥1 and in the intention-to-treat (ITT) groups. OS will be assessed in the subsequent interim analysis.
Findings will be announced at an upcoming medical conference and submitted to regulatory bodies. Currently, KEYTRUDA is authorized for use both as a standalone treatment and in combination therapies for suitable patients with metastatic or unresectable, recurrent HNSCC in the United States, Europe, China, Japan, and other regions globally. Further details can be found in the section titled “Selected KEYTRUDA® (pembrolizumab) Indications in the U.S.” below.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
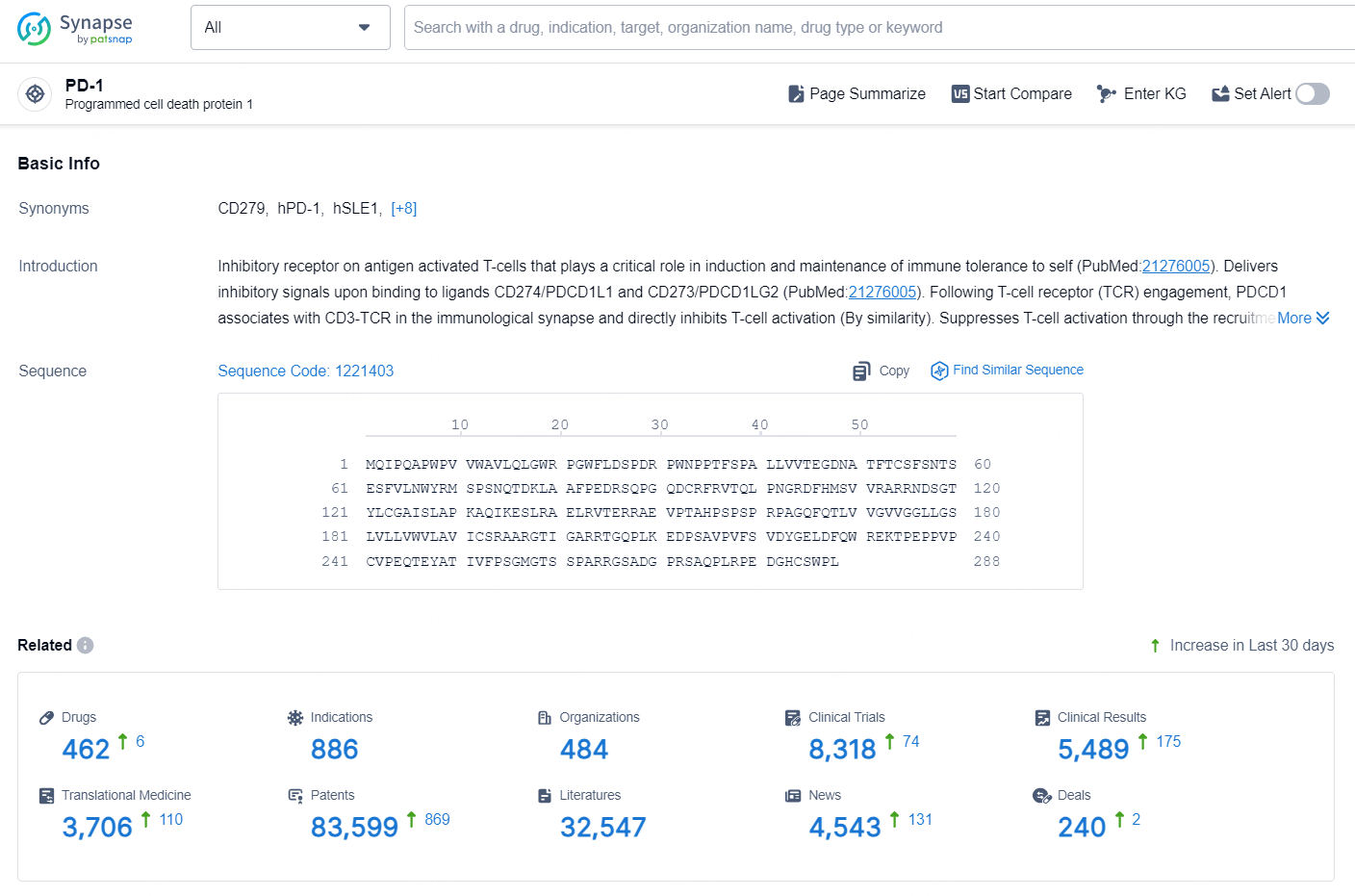
According to the data provided by the Synapse Database, As of October 11, 2024, there are 462 investigational drugs for the PD-1 target, including 886 indications, 484 R&D institutions involved, with related clinical trials reaching 8318, and as many as 83599 patents.
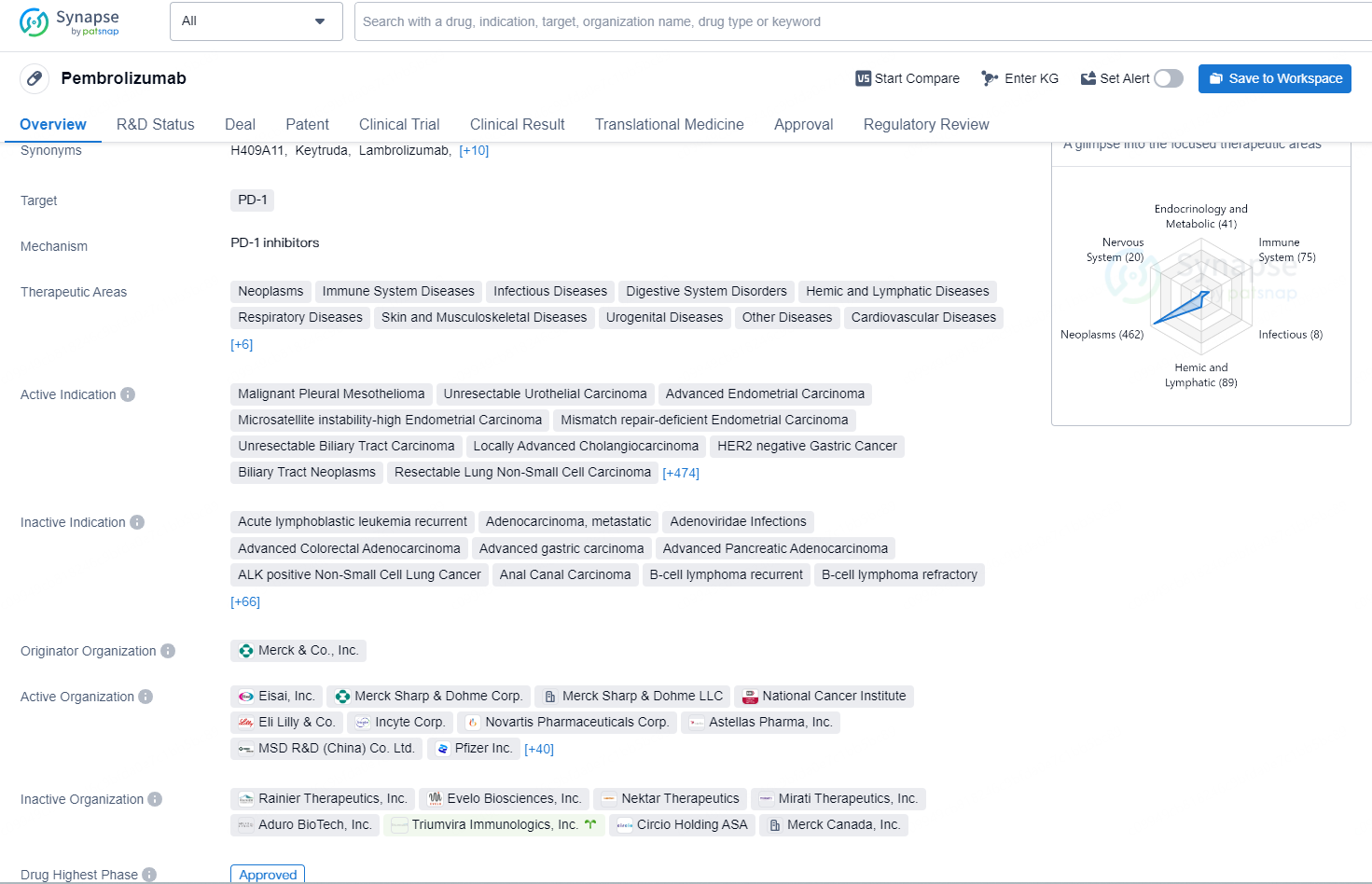
Pembrolizumab is a monoclonal antibody drug that targets PD-1 and is used in the treatment of a wide range of therapeutic areas including neoplasms, immune system diseases, infectious diseases, digestive system disorders, and many others. The drug is indicated for the treatment of various types of cancers, including but not limited to malignant pleural mesothelioma, unresectable urothelial carcinoma, advanced endometrial carcinoma, and HER2 negative gastric cancer. It has also been approved for use in the treatment of hepatocellular carcinoma, small cell lung cancer, and melanoma, among others.