Astellas and AviadoBio Sign Gene Therapy Deal for AVB-101 to Treat Frontotemporal Dementia
AviadoBio Ltd. and Astellas Pharma Inc. have disclosed an exclusive option and licensing agreement concerning AVB-101. This investigational gene therapy, which is based on AAV, is currently undergoing Phase 1/2 trials for individuals diagnosed with frontotemporal dementia linked to progranulin mutations (FTD-GRN).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
FTD is a severe form of early-onset dementia that usually results in death between three to 13 years after diagnosis. Individuals with FTD often undergo a swift deterioration in executive function, which includes aspects such as attention control, working memory, and problem-solving, along with unusual behaviors, loss of language skills, apathy, and diminished mobility. This condition is a significant contributor to dementia cases in those younger than 65 and is frequently overlooked or misdiagnosed.
According to the agreement, Astellas will have the opportunity to secure a global exclusive license for the development and marketing rights for AVB-101 concerning FTD-GRN as well as other possible indications. Astellas will invest $20 million in equity and provide up to $30 million in initial payments to obtain the option for licensing AVB-101. Additionally, AviadoBio stands to gain up to $2.18 billion in licensing fees and milestone payments, along with royalties, if Astellas decides to exercise its option.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
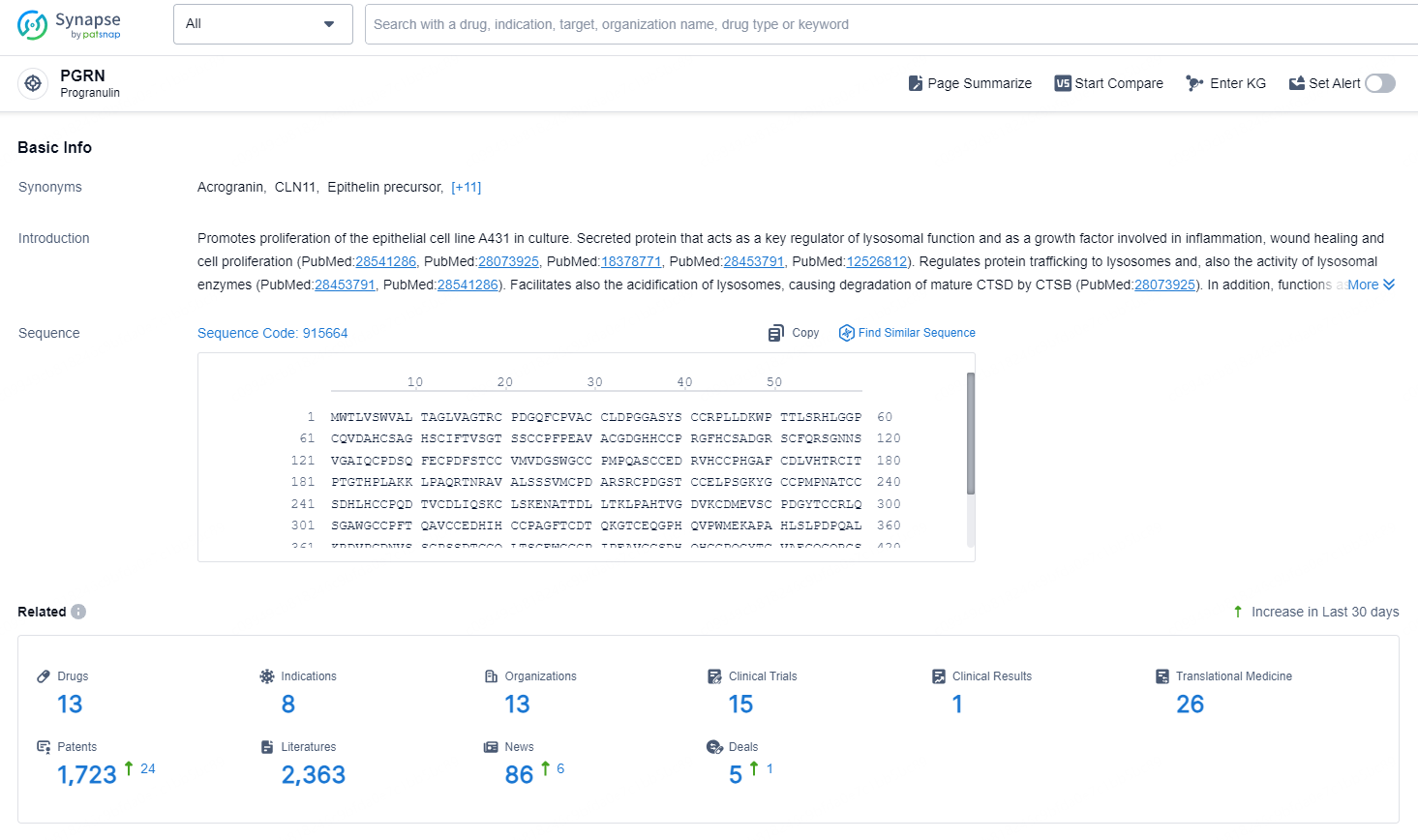
According to the data provided by the Synapse Database, As of October 11, 2024, there are 13 investigational drug for the PGRN target, including 8 indications, 13 R&D institutions involved, with related clinical trials reaching 15, and as many as 1723patents.
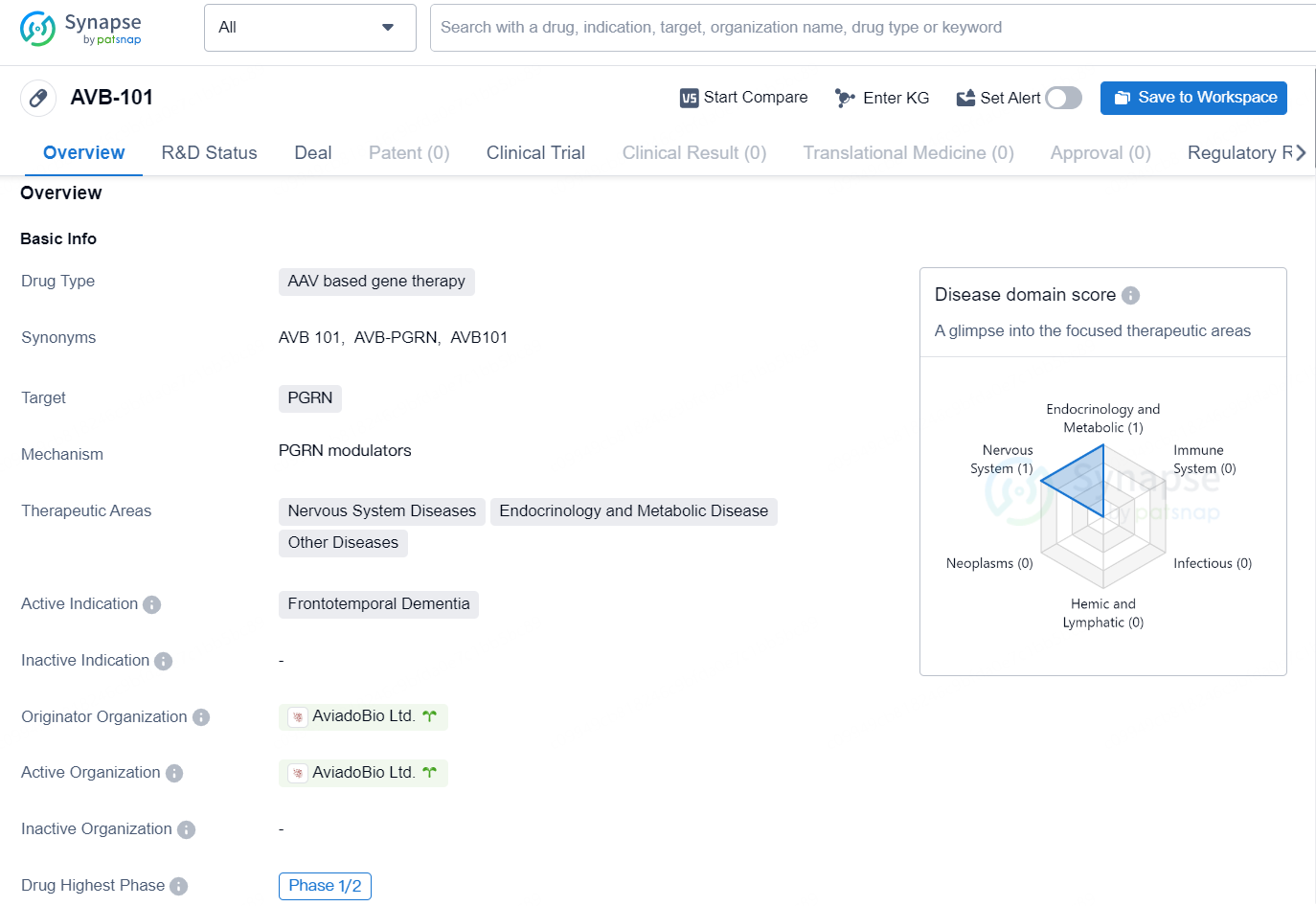
AVB-101 is an AAV based gene therapy developed by AviadoBio Ltd. The drug targets PGRN and is primarily focused on treating Frontotemporal Dementia, a type of neurodegenerative disease. Additionally, AVB-101 is also being explored for potential applications in the therapeutic areas of Nervous System Diseases, Endocrinology and Metabolic Disease, and Other Diseases.