ImmVira Discusses MVR-T3011 Clinical Data for High-Risk BCG-Failure NMIBC at ESMO 2024
ImmVira publicized the recent clinical outcomes for its primary oncolytic virus candidate, MVR-T3011, administered intravesically. This study targeted patients exhibiting high-risk, BCG-refractory non-muscle invasive bladder cancer (NMIBC). The findings were disclosed in a poster presentation at the 2024 European Society for Medical Oncology (ESMO 2024) conference.
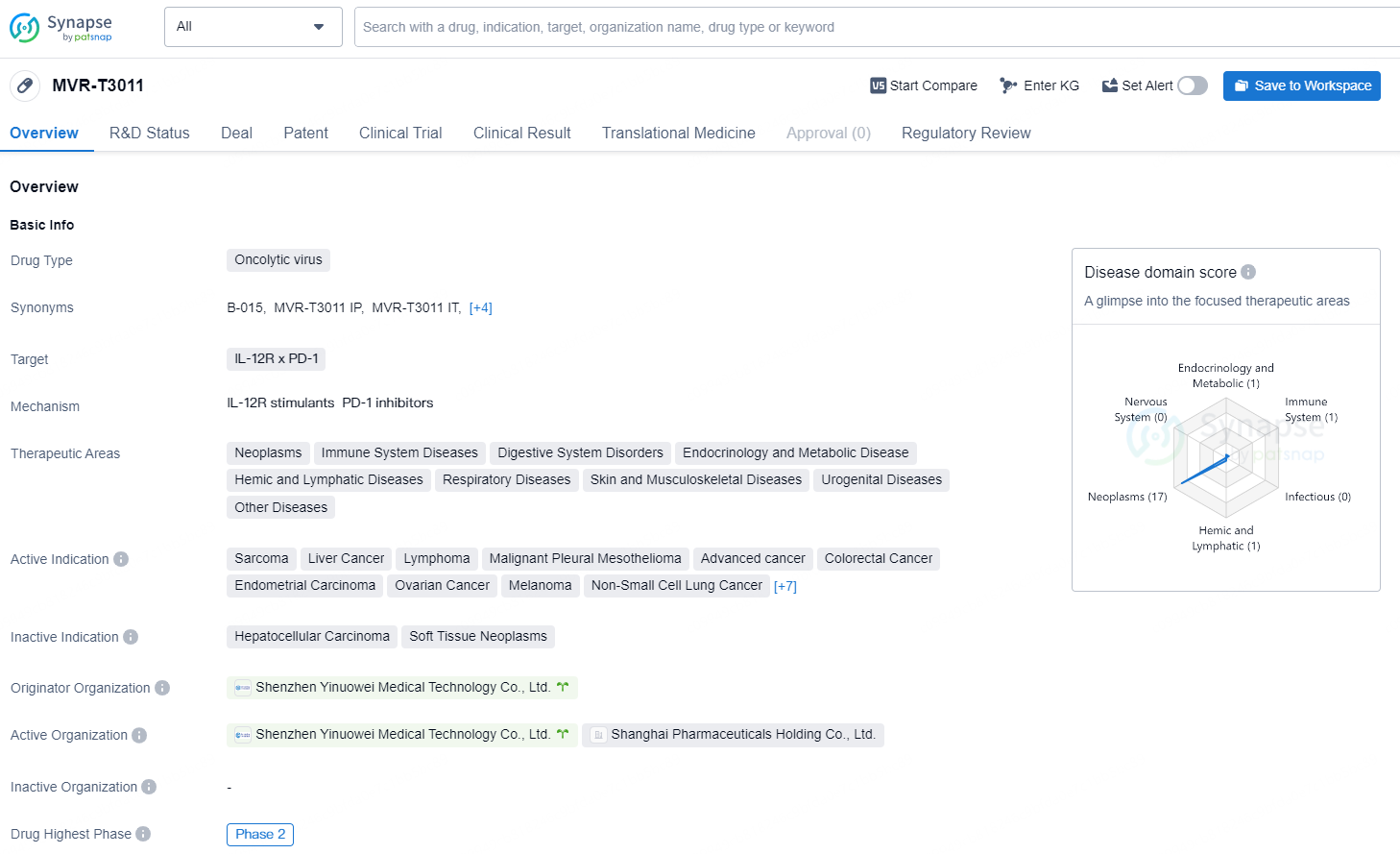
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This Phase I open-label study, involving dose-escalation and expansion phases, was performed in China to evaluate the safety and effectiveness of MVR-T3011 when administered intravesically to patients with high-risk BCG-failure NMIBC. The trial included a diverse patient demographic, encompassing those with high-grade Ta, T1, or CIS +/- Ta/T1 bladder cancer, irrespective of CIS status. The aim of this comprehensive approach is to engage a broader patient pool, potentially speeding up future trial enrollments and widening market prospects. Treatment was given through intravesical instillation at three varying dose levels, with a weekly induction course spanning 12 weeks and a bi-weekly maintenance course extending up to one year.
Based on data presented on a poster (cut-off as of June 27, 2024), among 14 evaluable patients, the overall 3-month complete response (CR) rate across all dose levels was 71.4% (10/14). At the 2×109 PFU dose level (anticipated RP2D), the CR rate impressively peaked at 87.5% (7/8). The study demonstrated a favorable safety profile, with no dose-limiting toxicities (DLTs) and no maximum tolerated dose (MTD) determined. Additionally, the MVR-T3011 treatment streamlines the clinical procedure by eliminating the need for a bladder prewash, thus reducing patient discomfort. The instillation process itself is both quick and efficient.
As of September 15, 2024, the latest data reveals that 20 subjects have undergone treatment with MVR-T3011. At the RP2D dose level, the 3-month CR rate remained high at 81.8% (9/11). Among the 9 patients who reached the 6-month evaluation, 8 maintained CR, and among the 4 patients who reached the 9-month evaluation, all continued in CR. Additional data collection is ongoing to support further analysis.
“There is a substantial unmet need for novel treatments for bladder cancer,” said Dr. Grace Zhou, Chairwoman and CEO of ImmVira. “We are very encouraged by the preliminary safety and efficacy data from this Phase I study, notably the 3-month CR rate in excess of 80%, along with sustained responses at the 6- and 9-month markers. With this early success, we are dedicated to expediting the clinical development of intravesical MVR-T3011 treatments for BCG-failure NMIBC patients and exploring its potential across other bladder cancer subtypes. Our objective is to offer a new, effective, and well-tolerated treatment option for patients.”
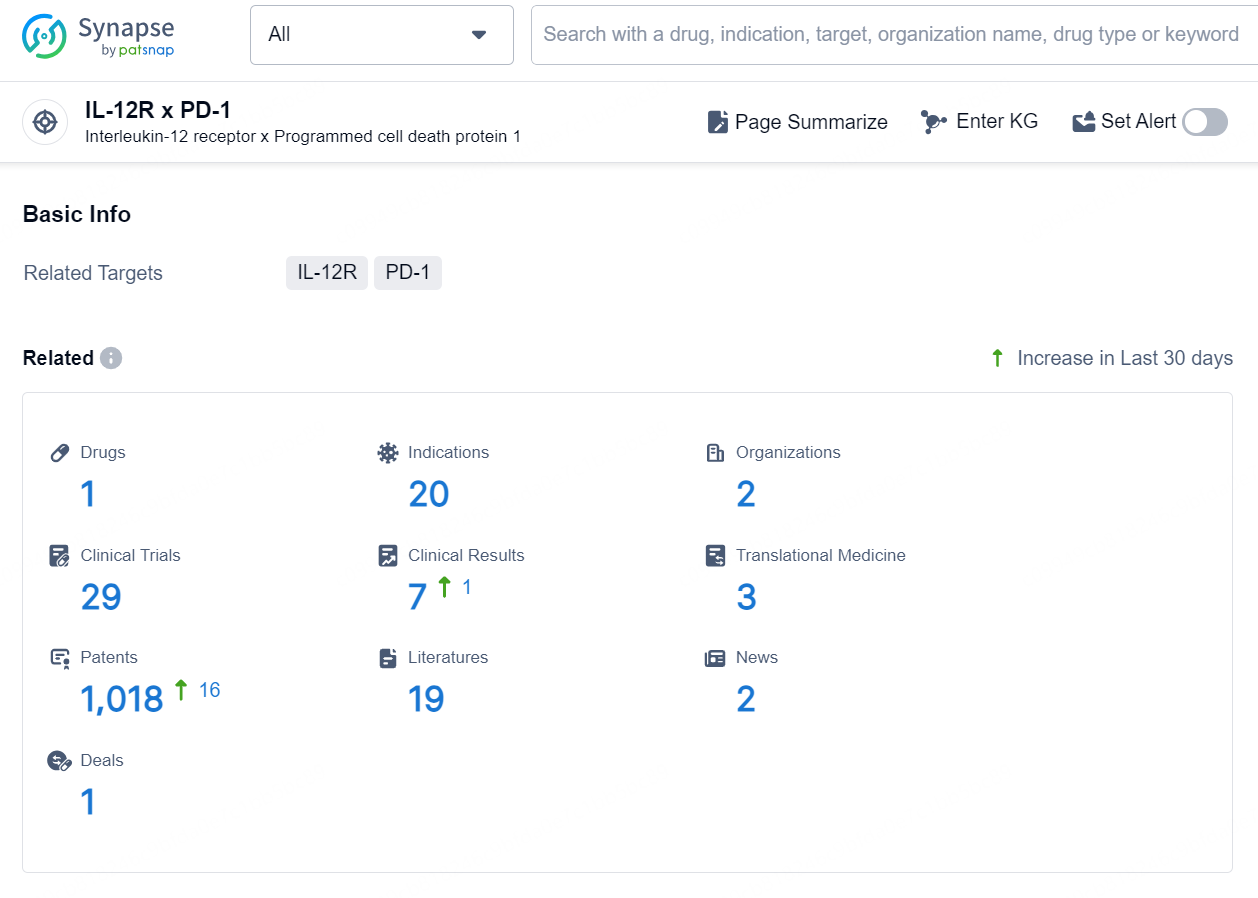
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 18, 2024, there are 1 investigational drug for the IL-12R x PD-1 targets, including 20 indications, 2 R&D institutions involved, with related clinical trials reaching 29, and as many as 1018 patents.
MVR-T3011 is an oncolytic virus drug developed by Shenzhen Yinuowei Medical Technology Co., Ltd., targeting IL-12R x PD-1. The drug is designed to treat a wide range of therapeutic areas, including neoplasms, immune system diseases, digestive system disorders, endocrinology and metabolic disease, hemic and lymphatic diseases, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases.