Tharimmune Partners with Intract Pharma to Develop Oral Infliximab, Secures Global Rights
Tharimmune, Inc. (NASDAQ: THAR) (“Tharimmune”), a biotechnology firm in the clinical development stage focused on creating therapeutic candidates for inflammation and immunology, revealed that it has secured a definitive agreement with Intract Pharma. Under this exclusive licensing deal, Tharimmune will obtain rights to INT-023/TH023, an oral monoclonal antibody against tumor necrosis factor-alpha (TNF-α), known as infliximab. This significant collaboration is designed to broaden Tharimmune’s therapeutic pipeline and strengthen its dedication to advancing novel treatments for autoimmune disorders.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
According to the agreement terms, Tharimmune has obtained the worldwide rights (excluding South Korea) to develop and market Intract Pharma’s delivery systems, Soteria® and Phloral®, as well as secured a pre-existing supply contract for infliximab to aid in the oral formulation development effort. Intract Pharma received an initial payment and stands to gain additional funds upon the Company’s equity financing. They will also benefit from future developmental, regulatory, and commercial milestones, in addition to mid-single digit royalties on the net sales of the product. The agreement maintains a first refusal right to proceed with development and commercialization after successfully completing a Phase 2 clinical trial. Tharimmune also has the opportunity to extend the license to Intract’s platform for up to four more targets.
Infliximab is a purified monoclonal antibody protein derived from recombinant DNA, featuring both murine and human elements that block TNF-α. TNF-α is a critical signaling protein linked to acute phase reactions and systemic inflammation. Janssen Biotech markets infliximab under the brand name Remicade® for various diseases, including Crohn’s disease, ulcerative colitis, rheumatoid conditions, and plaque psoriasis.
Administering antibodies like infliximab orally has traditionally been difficult due to the complexity of getting such large molecules through the gastrointestinal system. This new collaboration seeks to address these challenges by employing Intract’s delivery systems, potentially enabling infliximab to be taken in pill form. An oral delivery method has the potential to enhance patient adherence and quality of life, while decreasing the strain on healthcare systems caused by prolonged intravenous treatments.
“Incorporating Intract’s cutting-edge technology with the high-quality infliximab supplied through our existing agreement offers significant potential to expedite and strengthen our focus on immunology. This collaboration not only widens our therapeutic scope but also supports our mission to improve patient outcomes by creating more convenient and accessible treatments,” said Randy Milby, CEO of Tharimmune. “An oral form of infliximab marks a noteworthy progress in treating chronic inflammatory disorders and positions Tharimmune to compete in a large, global market.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
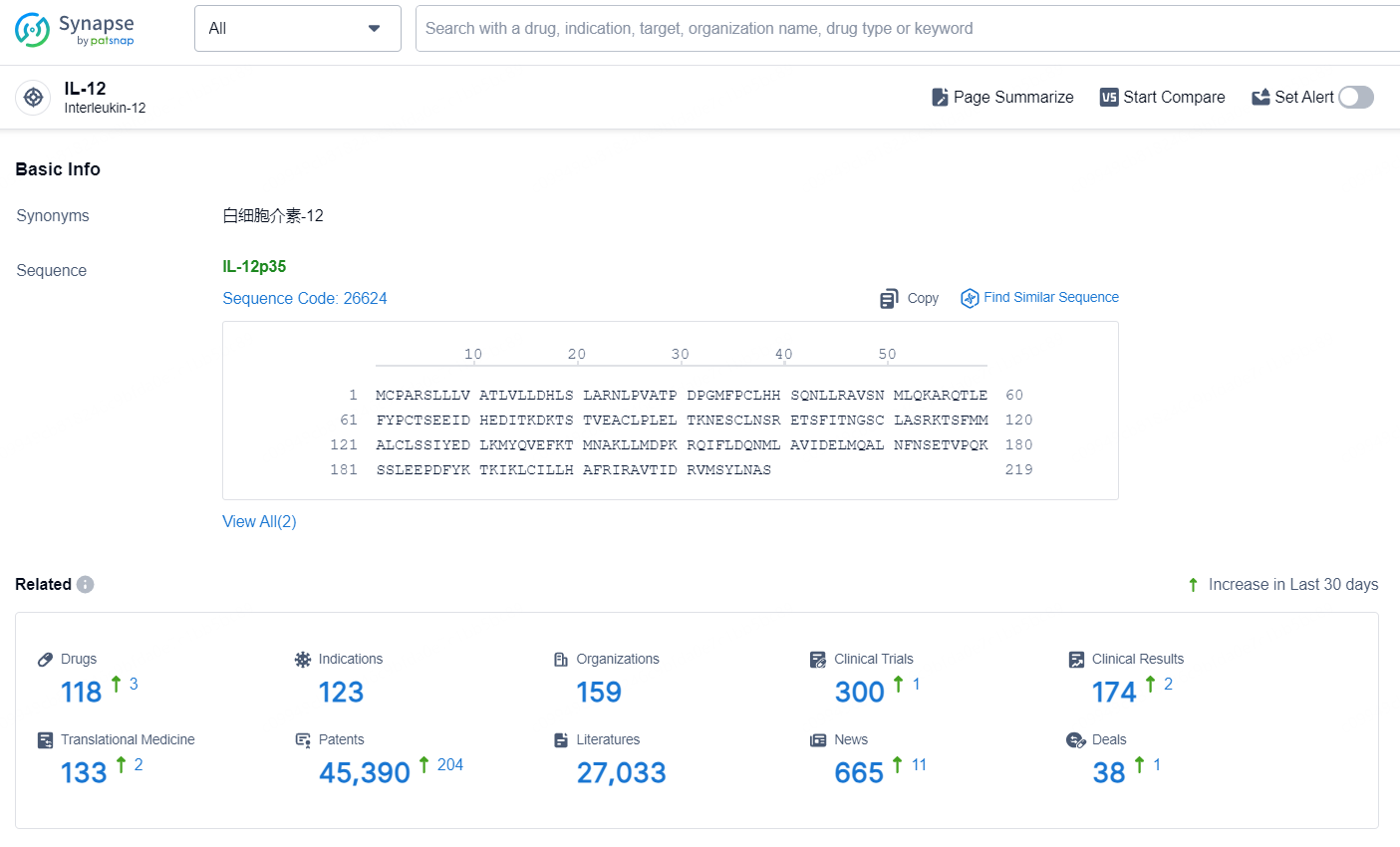
According to the data provided by the Synapse Database, As of September 18, 2024, there are 118 investigational drugs for the IL-12 target, including 123 indications, 159 R&D institutions involved, with related clinical trials reaching 300, and as many as 45390 patents.
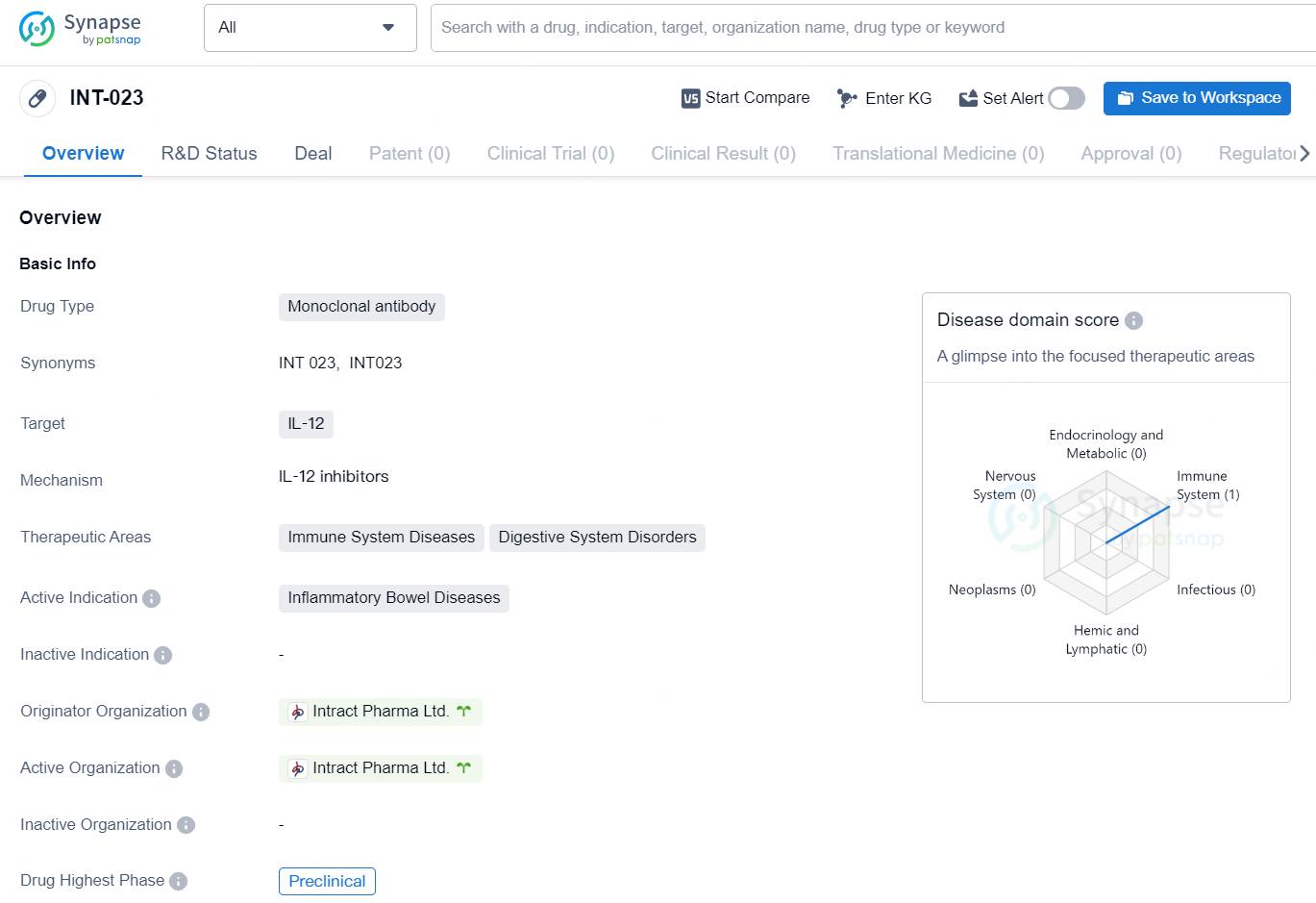
The drug INT-023 is a monoclonal antibody that targets IL-12 and is being developed by Intract Pharma Ltd. The therapeutic areas of focus for this drug are immune system diseases and digestive system disorders, with a specific active indication for inflammatory bowel diseases. Currently, the highest phase of development for INT-023 is preclinical.